1. Introduction
Biomorphic templating synthesis, due to its low cost and simple process, has been provided as an appropriate method to fabricate inorganic materials with predetermined microstructures. Up to now, various novel biomorphic materials with different functions have been developed using biotemplates such as protein,1) virus particles,2) diatoms,3) yeast,4) and butterfly wings.5) Functional materials with fibroid structure could provide high specific surface area and special properties, which can be used to develop new potential applications.
Ceria, as one of the most important rare earth oxide materials, has been extensively studied and applied in catalysis,6) gas sensing,7) solar cells,8) and solid oxide fuel cells9) due to its unique oxygen storage capacity (OSC), which originates from its ability to release and uptake oxygen under operation conditions.10) We have reported that filter paper has been employed as a biotemplate for the generation of micron braid structure CeO2 with high specific surface area of 71.3 m2·g−1 and enhanced catalytic activity.11)
A growing number of recent reports have been dedicated to ceria-containing solid solution or composite oxides, which could improve the physical/chemical properties of the ceriabased materials, such as the catalytic performance. Novel nanocrystalline Ce1−xLaxO2−δ (x = 0.2) solid solution was successfully prepared by Benjaram and exhibited a good catalytic property due to small crystallite size, facile reduction, profound bulk oxygen mobility, and enhanced OSC.12) Because of its potential to improve catalysts based on CeO2, CeO2-TiO2 composite has been provided with improved thermal resistance13) and better catalytic activity at lower temperatures. 14)
In the paper, using a filter paper template, we report the fabrication of CeO2-TiO2 composites with different Ce/Ti molar ratios. Meanwhile, the catalytic performance and formation of a micron braid structure were also investigated.
2. Experimental Procedure
The filter paper and all reagents were purchased from Sinopharm Chemical Reagent Co. Ltd. (SCRC). Typically, the solution was prepared by dissolving cerium nitrate hexahydrate (Ce(NO3)3· 6H2O, > 99.99%) in 100 ml deionized water with stirring for 30 minutes. The filter paper was cut into 10 mm × 10 mm squares; these they were soaked in the Ce(NO3)3·6H2O solution for 12 h. Afterwards, titanium tetrachloride (TiCl4, > 96%) was added, drop-by-drop, to the solution with proper agitation. The total of Ce(NO3)3·6H2O and TiCl4 was 0.01 mol, with Ce/Ti molar fractions of 10 : 0, 9 : 1, 8 : 2, and 7 : 3, for the samples designated as sample a, b, c, and d, respectively. The infiltrated paper samples were then dried overnight, and subsequently calcined in air at 500°C for 120 minutes to obtain CeO2-TiO2 composites.
The crystal structures of the samples were determined by power X-ray diffraction on a Rigaku D/MAX-2500PC diffractometer using Cu Kα radiation ( λ = 0.15406 nm). The XRD data were recorded for 2θ values between 20° and 60° at a scan rate of 0.020°·s−1. The surface structures of the samples were observed using a Hitachi S-4800 FESEM. Specific surface area and pore-size distribution were derived from the N2 adsorption/desorption isotherms obtained at −196°C on a Micromeritics ASAP-2010C instrument; the specific surface area was calculated using the Brunauer-Emmett-Teller (BET) method.
Temperature-programmed reduction (TPR) analysis was conducted on a TP-5000 (Tianjin Xianquan, China) analyzer with a TCD detector. The TPR profile of the powder (the sample had a weight of about 50 mg) was recorded between room temperature and 750°C at a heating rate of 10°C/min under 10% hydrogen flow in a nitrogen. For product analysis of a typical reaction, catalytic tests were conducted in a temperature-programmed reaction system equipped with a Shimadzu gas chromatograph (GC-14C); 100 mg product was loaded in a quartz reactor and heated from room temperature to 500°C at a heating rate of 2°C/min. The 40 mL/min gas flow was composed of 4% O2 and 2% CO, with N2 as balance gas.
3. Results and Discussion
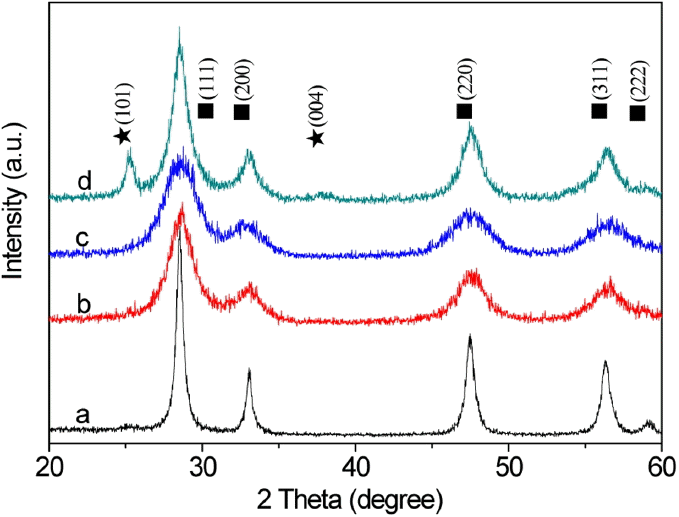
Figure 1 shows wide-angle XRD patterns of the CeO2-TiO2 composites with different Ce/Ti molar ratios after calcination treatment. The diffractograms of the samples shown in Fig. 1(a), (b), and (c) display only the XRD patterns of the face-centered cubic structure CeO2 (JCPDS 34-0394), indicating the formation of Ce-Ti oxide composite at molar ratios of 10 : 0, 9 : 1, and 8 : 2. No peaks at 27.5° could be observed, indicating the absence of anstase TiO2 phase.15) With increasing titanium content, a progressive shift of the diffraction peaks to higher Bragg angles is observed, because part of the smaller titanium species entered the ceriante lattice and provoked the contraction of the unit cell. A systematic width broadening of the peaks of the solid solution samples b and c is observed due to the occurrence of a more defective CeO2 lattice and smaller nanocrystals.16) When the molar ratio is 7 : 3, a peak at 27.5° appears in Fig. 1(d); this peak is attributed to anstase structure TiO2; however, most of the powders retained the cubic phase and single solid solution structure, indicating the existence of separated CeO2-based oxide solid solution and TiO2.15)
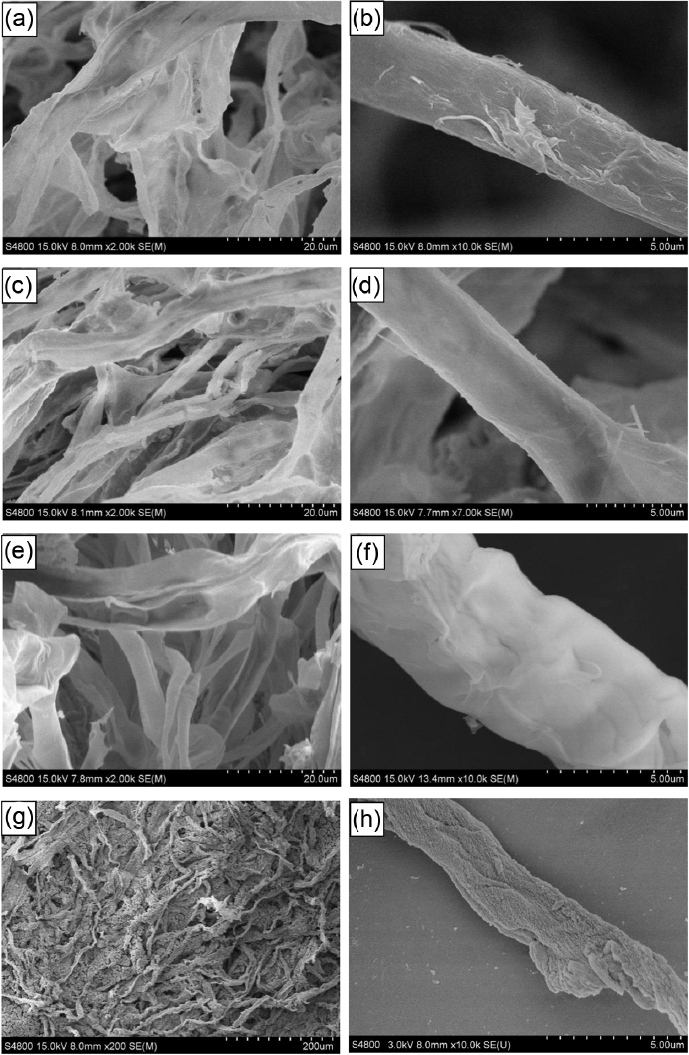
Figure 2 shows FESEM images of the synthesized pure CeO2 and CeO2-TiO2 composites with different Ce/Ti molar ratios. As can be seen in Fig. 2(a) to (f), the replicas completely mimic the shape of the paper template and exhibit micron braid microstructures constituted of randomly arranged fibrous structures about 1 - 6 μm in diameter with lengths ranging from 50 to 200 μm. The edges of samples a, b, and c are smooth and show orientation flow lines, which illustrates that the products perfectly replicate the micron braid fiber structure of the filter paper. Fig. 2(g) is an FESEM photo of the Ce/Ti molar ratio 7 : 3 sample. From this image, it can be seen that the micron braid long fibrous structure collapsed and reunified. It can be seen from the high magnification SEM morphology (Fig. 2(h)) that the surface of the product is made up of tiny nanoparticles that are caused by titanium oxide particles precipitated from the CeO2-TiO2 solid solution. This result is in good agreement with the XRD analysis.
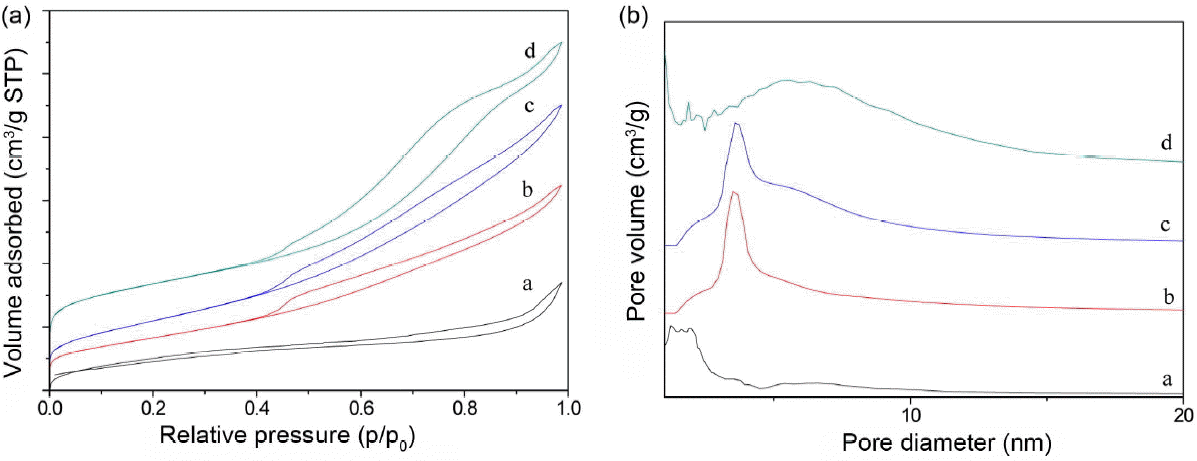
In Fig. 3, the N2 adsorption-desorption isotherms and Barrett-Joyner-Halenda (BJH) pore-size distribution curves provide further information about the structure of the samples. Fig. 3(a) shows that the curves of the N2 adsorption-desorption isotherms for all samples are similar and are intermediate types between types I and IV, according to the IUPAC classification.17) The shapcurve shapes indicate the existence of micropores in addition to mesopores.18) The pore size distributions (Fig. 3(b)) are concentrated below 10 nm, indicating that these pores are nanoporous scope. It can be observed that the BJH pore size distribution for samples b and c has narrow pore distributions covering a range of about 4 nm. A visible shift can be noted for the other samples, since the narrow pore distribution has disappeared. The surface areas of all samples, calculated via Brunauer-Emment-Teller (BET) analysis, are shown in Table 1. The sample with the Ce/Ti molar ratio of 8 : 2 has the largest surface area at 81.0 m2·g−1; this is probably attributable to the positive physicochemical contribution of and good thermal resistance arising from the addition of Ti ion dopant to the form sosoloid aligned with the Ce4+ and Ti4+ species. These results further confirm the above information on the structure of the samples.
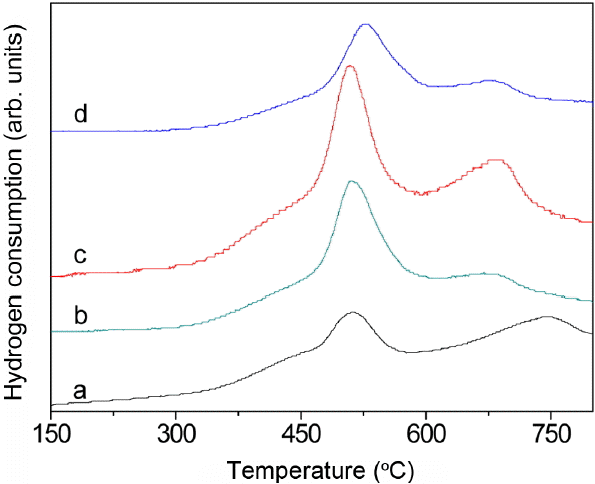
The redox properties of the samples were investigated by H2-TPR experiments. Fig. 4 shows the H2-TPR result for CeO2-TiO2 composites synthesized using the paper template. The pure ceria (Fig. 4(a)) demonstrates reduction peaks at about 520°C and 750°C due to surface and bulk reduction, respectively.19) The relative peak intensity at 750°C in pure ceria demonstrates that bulk oxygen reduction is the main process (Fig. 4(a)). As observed in the TPR plots of the CeO2-TiO2 solid solution, the reduction temperatures for samples b and c are 500°C and 670°C for the surface and bulk oxygen reductions, respectively. Doping of ceria with Ti ion leads to shifting of the reduction peaks toward a lower temperature, and the total amount of active oxygen from the solid solution sample is about 2-3 times higher than it is for the pure ceria. The low temperature surface reduction of the CeO2-TiO2 solid solution can also be partly attributed to the increasing surface area discussed in the previous section. The reason for the low temperature of the bulk reduction could be that the diffusion of bulk oxygen to the surface is relatively fast, creating more active sites on the surface for the adsorption of H2.20) Hence, with an increase of Ti molar rate, a shift of the surface reduction peak toward higher temperatures takes place due to the decrease of the surface area and the generation of TiO2 particles. Decrease in the surface is expected to reduce the number of active sites for H2 adsorption, thereby decreasing the consumption of bulk oxygen.
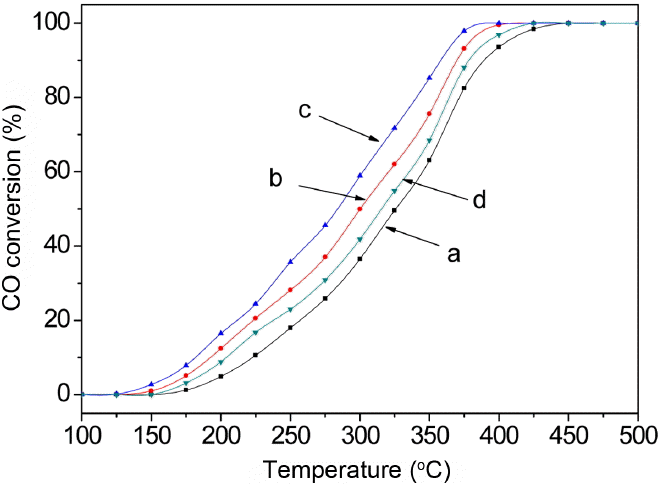
Figure 5 shows the catalytic activity profiles of the micron braid pure CeO2 and CeO2-TiO2 oxide composites; the reaction is as follows: 2CO + O2 = 2CO2. The surface area of pure CeO2 is 61.2 m2·g−1. Moreover, the surface area of the CeO2-TiO2 composites is larger than that of pure CeO2. From the figure, it can be seen that the CO oxidation activity of the CeO2-TiO2 composite is obviously higher than that of CeO2. Sample c with the Ce/Ti molar ratio of 8 : 2 shows a notably lower conversion temperature than those of other materials. For example, at 280°C, the conversion rate of sample c is above 50%, while that of the sample of pure CeO2 is below 25%. A 100% CO conversion of sample c was obtained at 400°C. This could be due to the fact that the formation of solid solution may give rise to more oxygen vacancies, which situation has been confirmed in the H2-TPR test. Moreover, the high surface area at this optimal mole fraction is believed to contribute to the enhanced catalytic performance. However, the results in this study suggest that the doping ion effect is not linear. Excessive doping of Ti4+ into CeO2 results in the formation of mixed phases, and the resulting product has lower surface area and worse catalytic activity than those characteristics of the solid solution sample.20)
4. Conclusions
In summary, micron braid structure CeO2-TiO2 composites with different Ce/Ti molar ratios have been successfully synthesized using filter paper as biotemplate. The fibers of this product structure are about 1-6 μm in diameter, with lengths ranging from 50 to 200 μm. The synthesized composites show higher surface oxygen activity at low-temperature and larger surface area than those characteristics of pure ceria; these results has been discussed in detail after TPR analysis and N2 absorption-desorption analysis. The solid solution product at a Ce/Ti molar fraction of 8:2 displays superior catalytic activity for CO oxidation, which at about 280°C was above 50% and at temperatures below 400°C was 100%, suggesting the potential of this material for use in catalysis, sensing, auto-gas purification, etc.