Cao, Song, Xiang, Hu, Zhang, Xia, Xu, Zha, Li, Gonzale, Han, and Chen: Modeling, Preparation, and Elemental Doping of Li7La3Zr2O12 Garnet-Type Solid Electrolytes: A Review
Abstract
Recently, all-solid-state batteries (ASSBs) have attracted increasing interest owing to their higher energy density and safety. As the core material of ASSBs, the characteristics of the solid electrolyte largely determine the performance of the battery. Thus far, a variety of inorganic solid electrolytes have been studied, including the NASICON-type, LISICON-type, perovskite-type, garnet-type, glassy solid electrolyte, and so on. The garnet Li7La3Zr2O12 (LLZO) solid electrolyte is one of the most promising candidates because of its excellent comprehensively electrochemical performance. Both, experiments and theoretical calculations, show that cubic LLZO has high room-temperature ionic conductivity and good chemical stability while contacting with the lithium anode and most of the cathode materials. In this paper, the crystal structure, Li-ion transport mechanism, preparation method, and element doping of LLZO are introduced in detail based on the research progress in recent years. Then, the development prospects and challenges of LLZO as applied to ASSBs are discussed.
Key words: Li7La3Zr2O12, inorganic solid electrolyte, garnet structure, element doping, Li-ion transport mechanism, synthesis methods, all-solid-state batteries
1. Introduction
To meet the increasing energy demand, more efficient and safer electrochemical energy storage has become a hot spot in related fields. With their high energy density and favorable cyclic stability, Li-ion rechargeable batteries (LIBs) stand out from other electrochemical energy storage devices and have been widely used in power supplies such as electrified transportation and portable electrical devices, 1,2) However, despite their high lithium-ion conductivity, conventional lithium-ion batteries with liquid electrolytes may result in excessive charging, internal short circuiting, and other anomalies that lead to serious consequences such as leakage, self-ignition or even explosion. In comparison to LIBs, ASSBs with solid electrolytes show great prospects and can be considered as the next generation of devices for energy storage on account of their comparatively excellent properties: higher safety, longer cycle life, higher energy density and power density, fewer requirements for packaging, lower self-discharge rate, state-of-charge monitoring circuits, and no memory effect. 3-5) To meet the requirements for the successful fabrication and commercialization of all-solid-state lithium secondary batteries, solid electrolyte which is the key influencing component, should have a wide electrochemical window, high lithium-ion conductivity, and good chemical and electrochemical stability for lithium. 6)
Nowadays, the mainly researched solid electrolytes are polymer electrolytes and inorganic ones. As the most representative polymer electrolytes, poly(ethylene oxide) (PEO)- based electrolytes show very good solubility for lithium salts as well as good flexibility and tensile shear properties. However, their low ionic conductivity at ambient temperature and narrow electrochemical window are insufficient for meeting practical requirements. 7) By contrast, inorganic electrolytes have many advantages including relatively high ionic conductivity, high-level safety, and wide electrochemical window. The research studies of inorganic electrolytes have mainly concentrated on NASICON, garnet, LISICON, perovskite, LiPON, and sulfide electrolytes, but most of them cannot meet all of the needs above. NASICON- type electrolytes, which are stable in air and water and have high lithium-ion conductivity, allow the migration of the charge carriers in all three dimensions. A typical NASICON-type electrolyte Li 1.3Al 0.3Ti 1.7(PO 4) 3 whose skeleton is most suitable for ionic migration possesses a high ionic conductivity of 7 × 10 −4 S/cm at 25°C. 8) However, these kinds of electrolytes still show drawbacks of bad interfacial contact as well as being unstable with Li metal owing to facile Ti 4+ reduction. 9,10) With the same drawbacks as the NASICON-type, 11) the perovskite-type electrolyte can achieve a grain conductivity close to 10 −3 S/cm, but the total conductivity is relatively low owing to the high boundary resistance. 12,13)
Existing scientific research shows that the ionic conductivity has a tangible correlation with the formation enthalpy. 14) LISICON-type electrolytes can show great stability even with long cycling and high rate capability, 15) but in contrast to the above two types of electrolytes, the LISICON-type ones have relatively low lithium-ion conductivity that decreases further with time. These electrolytes are also unstable to Li metal and atmospheric CO 2. 9,16) Although LiPON-type electrolytes show great compatibility with positive and negative poles, high mechanical stability, and a broad electrochemical stability window, the low conductivity of about 2.3 × 10 −6 S/cm at room temperature cannot be ignored in applications. 17,18)
The sulfide-type electrolyte Li 9.54Si l.74P 1.44S 11.7Cl 0.3 has a lithium-ion conductivity of about 10 −2 S/cm at ambient temperature. 19) These electrolytes also show good performance in thermostability and softness. Nonetheless, the bad contact and stability with both poles as well as the release of H 2S or other harmful gas after reacting with water and oxygen in the air cause adverse impacts on the further research and applications of sulfide-type electrolytes. 9,20)
The typical garnet-type material Li xLa 3M 2O 12 (M = Ta, Nb, Zr) shows numerous advantages such as high lithiumion conductivity, negligible electronic transport, good thermal and chemical stability against Li metal, and a wide electrochemical window. With better performance, the traditional garnet-type electrolyte Li 7La 3Zr 2O 12 (LLZO) shows high Li-ion conductivity of 0.1-1 mS/cm at ambient temperature and a wide electrochemical window of above 5 V after adulteration. 21) Since 2007, garnet-type Li 7La 3Zr 2O 12 was firstly reported by Murugan using a conventional solid-state reaction with an ionic conductivity of 3.0 × 10 −4 S/cm, 22) many studies of LLZO have been done on account of its high conductivity and the higher possibility of synthesizing the ideal electrolyte. In addition, many studies were conducted to obtain electrolytes with excellent properties by modifying LLZO. 23-27) As a result of a comprehensive comparison, LLZO is considered one of the most promising electrolytes to meet the requirements of ideal electrochemical energy storage devices. 28)
In this review, we first analyze the crystal structure, phase transition process, and lithium ion migration mechanism in LLZO. Then, different synthesis methods and their corresponding characteristics are introduced in detail. Finally, we summarize some research progress in the element doping of LLZO in recent years and present some perspectives.
2. Crystal Structure and Lithium Diffusion Mechanism of Li-stuffed Garnet
Generally, lithium garnet shows two crystal structures: tetragonal and cubic phase. The two garnet phases have the same framework structure but differ in lithium distribution, which strongly affects the lithium diffusion mechanism and electrochemical performance of the two garnet phases. For a better understanding of lithium garnet, this section introduces the crystal structure of the two garnet phases, after which the theoretical and experimental approaches to the phase transition process and lithium migration mechanism are discussed.
2.1. Crystal structure
A fast Li + conductor with a garnet-like structure was first reported by Prof. Werner Weppner’s Group, 29,30) The lithium garnet family exhibits the general formula of A 3B 2C 3O 12 (A = La 3+, Y 3+, Nd 3+; B = Te 6+, Ta 5+, Nb 5+, Zr 4+), where A and B are eight- and six-coordinated sites. The framework structure is composed of edge-sharing [AO 8] dodecahedron and [BO 6] octahedrons. Lithium ions occupy the interval tetragonal and octahedral sites. These lithium polyhedrons interconnect to form a lithium loop structure as shown in Fig. 2, which is the basic lithium diffusion pathway in a lithium garnet solid electrolyte. 31) Commonly studied lithium garnets contain three to seven per formula and are usually crystallized in a cubic structure with an Ia3̄ d space group. 30-32) However, Awaka et al. 33) found that Li 7La 3Zr 2O 12(LLZO) prefers crystallization in a tetragonal phase with I4 1/ acd at room temperature and exhibits a lithium ionic conductivity that is two orders of magnitude lower than that of the cubic phase. A less conductive tetragonal phase also exists in other lithium garnets with seven Li + per formula, such as Li 7La 3Sn 2O 12 34) and Li 7La 3Hf 2O 12. 35) The crystal structures of tetragonal LLZO (t-LLZO) and cubic LLZO (c-LLZO) are shown in Fig. 1. t- and c-LLZO have the same framework structure but differ in their lithium distributions. In the cubic phase, lithium disordering is observed in partially occupied c-24d (tetragonal site) and c-96h (octahedral site) sites. In the tetragonal phase, t-8a (tetragonal site), t-16f, and t-32g (octahedral site) are fully occupied by lithium ions, leaving t-16e (tetragonal site) unoccupied. This results in the complete ordering of lithium ions and a Li-Li distance longer than 2.5 Å. 31)
2.2. Phase transition
Depending on the synthesis and simulation methods, the phase transition temperature varies from 450 to 1000 K. 36-40) Bernstein et al.37) stated that the tetragonal phase stability gains from the lithium-ion sublattice ordering and volume-preserving tetragonal distortion, which increase the Li-Li distance and relieve the internal structural strain. Therefore, the introduction of a lithium vacancy is necessary for the stabilization of the cubic phase. Adams et al. 40) performed a molecular dynamic simulation and in-situ XRD to study the phase transition in lithium garnets. The results, as shown in Fig. 3, confirmed that the cubic phase can be stabilized by pentavalent doping, which increases the lithium vacancy concentration and reduces the local lithium-ion ordering. From ordering to disordering, the phase transition from t- LLZO to c-LLZO is accompanied with a lithium redistribution among all lithium sites. Bernstein et al. 37) suggested that lithium redistribution is simply a shift from t-8a to t- 16e based on the unchanged occupancy of c-24d (c-24d = t- 8a + t-16e). Klenk et al.38) studied the evolution of the cage occupancy for four different lithium sites (t-8a, t-16e, t-16f, and t-32g) with temperature. The results are shown in Fig. 4. They concluded that lithium redistributes among all tetragonal sites. In addition, the phase transition initiates at the t-8a site with the cooperation of the t-32g site. Chen et al.41) calculated the defect energy of four lithium vacancy models and concluded that the lithium redistribution is determined by the site energy preference. The lithium vacancy located at the t-32g site exhibits the lowest energy among the t-8a, t-16f, and t-32g sites, which leads to the initiation of Li redistribution on the t-32g site. Then, lithium ions at the t-8a and t-16f sites participate in the redistribution one after another. In conclusion, the phase transition from t-LLZO to c-LLZO is a site-energy-preference- determined, entropy-driven process, the physical expression of which is the redistribution of lithium ions from the t-32g, t-8a, and t-16f to t-16e sites.
2.3. Lithium diffusion
The difference in lithium distribution between t-LLZO and c-LLZO results in reduced lithium diffusion properties of t-LLZO compared to c-LLZO. Many research studies have been carried out to reveal the relationship between the lithium arrangement and the lithium diffusion mechanism. Meier et al.42) performed first-principles-based calculations to study the lithium diffusion mechanism in both t-LLZO and c-LLZO as shown in Fig. 5. The results suggested that lithium diffusion exhibits a fully collective motion in t-LLZO and single-ion jumps in c-LLZO. The latter mechanism exhibited a relatively lower energy barrier. Jalem et al. 43) studied the lithium diffusion in c-LLZO by molecular dynamic calculation. A concerted migration mechanism for lithium diffusion was also found in c-LLZO. A classical molecular dynamic simulation performed by Klenk et al. 38,39) suggested that lithium diffusion exhibits oscillations in low-temperature t-LLZO and “structured diffusion” in high-temperature c-LLZO. The researchers also calculated the Haven ratio (the ratio of self-diffusion vs. collective diffusion), which increased from 0.05 to 0.35 when the temperature increased from 700 to 1400 K. The results of the Haven ratio calculation illustrated that the collective diffusion exists in both t- and c-LLZO, but varies in degree. By combining a lithium local structure and the evolution of the site occupation with temperature, Chen et al. 41) found a temperature-dependent lithium migration pathway. Lithium ions exhibited uniformly three-dimensional diffusion in c-LLZO, which is the same as in other reports. By contrast, in t-LLZO, an unusual two-dimensional-like lithium diffusion mechanism was observed. The decrease of the migration pathway with regard to its dimension is responsible for the low lithium diffusion properties in t-LLZO. Chi Chen et al. 44) combined machine learning and information theory to analyze molecular dynamic trajectories, and found that c- LLZO has a higher hopping frequency than t-LLZO. In addition, lithium exhibits uncorrelated Poisson-like diffusion in c-LLZO and correlated diffusion in t-LLZO as shown in Fig. 6. This work introduced a unique perspective in analyzing molecular dynamic results.
3. Synthesis Methods
LLZO can be obtained by different synthesis methods: conventional solid-state reaction, sol-gel method, field-assisted sintering, 45-48) coprecipitation, 48,49) electrospinning, 50) and spray pyrolysis synthesis. 51)
3.1. Conventional solid-state reaction
As the most widely used method to prepare LLZO, the conventional solid-state reaction has several advantages: a simple preparation process, suitability for a large yield, low cost, etc. The grinding and sintering process in this method needs to be repeated in order to enhance the performance of products. The purpose of the first round of grinding and sintering is to mix the raw materials and obtain the early LLZO phase, respectively. The purpose of the second round of grinding and sintering is to obtain a uniform particle size and the final product, respectively.
The first report on LLZO was published by Murugan et al. in 2007. 30) The author used a conventional solid-state reaction to prepare Li 7La 3Zr 2O 12 at 1230°C for 36 h. The Li + conductivity was 3 × 10 −4 S/cm. Many research studies on LLZO were conducted afterward. However, impure phases are introduced to systems easily via the conventional solid-state reaction because the high temperature and long sintering time cause too much lithium loss, and other elements diffuse from the crucible. Kai Liu et al. 53) prepared Li 6.5La 3- Ta 0.5Zr 1.5O 12 in an alumina crucible and LiAlO 2 appeared as a second phase since the Al 3+ diffused from the alumina crucible to the LLZO. Rangasamy et al. 52) prepared Al-doped LLZO with various Li + concentrations by adding different amounts of lithium sources. However, there was La 2Zr 2O 7 and LaAlO 3 in the samples. The XRD of the samples is shown in Fig. 7. To prepare pure LLZO samples, mother powders were placed on an alumina crucible to prevent the pellets from being contaminated by Al 3+. Different amounts of lithium sources were adopted to compensate for the lithium loss during calcination. In addition, in order to obtain LLZO with higher Li + conductivity and a more stable structure, researchers adjusted the types of raw materials, lithium content, process parameters (sintering temperature, pressure, time, etc.), and various dopants. Allen et al. 54) used Li 2CO 3, La(OH) 3, and ZrO 2 as raw materials, which were calcined at 1000°C for 3 h to obtain LLZO with a Li + conductivity of 3.7 × 10 −4 S/cm. Its density was as high as 98%, while the activation energy was only 0.30 eV. Lee et al. 55) used LiOH, La 2O 3, and ZrO 2 as raw materials. After high-energy ball milling for 12 h, the pellets were calcined at 1125°C for 20 h to obtain LLZO with a Li + conductivity of 4.9 × 10 −4 S/cm at room temperature. Yutao Li et al. 56) used 0.6 mol of Ta-doped Zr sites and prepared Li 6.4La 3Zr 1.4Ta 0.6O 12 in an alumina crucible. The ionic conductivity was 1.0 × 10 −3 S/cm. Jianli Gai et al. 57) reported on Li 7La 3ZrNb 0.5Y 0.5O 12 prepared by co-doping Nb and Y on a Zr site, for which the ionic conductivity was 8.29 × 10 −4 S/ cm. Xin Guo et al. 58) used a Ga-doped Li site to obtain Li 6.24Ga 0.25La 3Zr 2O 12. The sample exhibited excellent Li + conductivity as high as 1.46 × 10 −4 S/cm at room temperature. Yiqiu Li 59) prepared Li 6.75La 3Zr 1.75Ta 0.25O 12 in different atmospheres and found that O 2 contributes to the growth of grains and enhances the Li + conductivity. A sample sintered in O 2 exhibited a maximum Li + conductivity of 7.4 × 10 −4 S/ cm at room temperature.
Table 1 summarizes the cubic phase LLZO prepared by the conventional solid-state reaction that was reported.
3.2. Sol-gel method
In order to get LLZO with a smaller particle size, more uniform distribution, and lower sintering temperature, the sol-gel method is usually employed. The sol-gel method can make the components uniformly mix in the liquid phase and react with each other to obtain a uniform precursor powder. Then, the precursor powder will be sintered at a relatively low temperature to prepare a nanosized LLZO powder. 68) This is beneficial for the preparation and performance optimization of inorganic solid electrolytes. Xie et al. 69) and Kokal 70) successfully synthesized pure-cubic-phase LLZO nanopowders by the sol-gel method at a low temperature of 750°C, but the Li + conductivity was only 10 −6 S/cm at room temperature. Sakamoto et al. 71) used LiNO 3, La(NO 3) 3·6H 2O, and Zr(OH 7C 3) 4 as raw materials and acetic acid as chelating agent. The wet gel was dried by a supercritical drying technique and then sintered at 1000°C for 4 h. The nano-cubic phase LLZO with a grain size of only 260 nm had a high Li + conductivity (4 × 10 −4 S·cm −1) at room temperature. I. Kokal 70) prepared LLZO, and the total Li + conductivity was 3.12 × 10 −7 S/cm. Fig. 8 shows SEM micrographs of the LLZO powder precursors calcined at different temperatures. It can be seen that the size of the precursors is on the nanoscale. Reports on the preparation of LLZO by the solgel method in recent years are listed in Table 2.
3.3. Field-assisted sintering method
Field-assisted sintering technology (FAST) is a new type of technology for the rapid sintering preparation of LLZO. This process uses uniaxial pressure and passes direct current through a mold. This method is similar to hot pressing (HP). In comparison with hot pressing, where the heat generation occurs externally, the heat is produced internally for FAST. The raw material powder is placed in a mold made of graphite or other materials, and the heat is transmitted by conduction to the powder. Because of the good electrical conductivity of materials used for the mold, high current can be achieved at low voltage. A spark discharge removes impurities from the surface of the particles and activates the surface to promote sintering, which is beneficial to the low temperature, rapid sintering, and densification of the raw material powder. 79) Zhang et al.45) used field-assisted sintering technology to prepare LLZO with a high density of 99.8% for the first time at 1100°C. The sintering time was only 10 min, and the Li + conductivity was 5.7 × 10 −4 S/cm at room temperature, which is the highest among the same materials. In order to increase the production scale of the laboratory, Botros et al. 46) combined spray pyrolysis (NSP) and field-assisted sintering to reduce the sintering temperature to 950°C. The Li + conductivity was 3.3 × 10 −4 S/cm. Zhang et al. 48) prepared the precursor powder of a nano-nuclear- shell structure by the coprecipitation method, and then reduced the sintering temperature to 900°C by field-assisted sintering technology. Finally, they obtained the LLZO with a particle size of 1-3 μm. The Li + conductivity was 3.32 × 10 −4 S/cm at room temperature with a density of 96.5%. Since the sintering time of the field-assisted sintering method is very short, the lithium loss during sintering can be effectively reduced. The density of the material can be improved by applied uniaxial pressure during sintering. Thus, the field-assisted sintering method is particularly suitable for rapidly preparing high-purity, high-density LLZO electrolyte materials. A schematic diagram of FAST is shown in Fig. 9. SEM micrographs of cross sections of the samples are shown in Fig. 10. These samples were sintered at (a) 1150°C and (b) 1200°C. The SEM images revealed good interfacial bonding between the particles. There were no intergranular fractures and almost no voids.
Table 3 shows a comparison between FAST and the two abovementioned preparation methods. In this table, the advantages of FAST can be clearly seen.
3.4. Other preparation methods
In addition to the abovementioned methods, new preparation methods of LLZO have been studied. These include coprecipitation, electrospinning, and spray pyrolysis. Every method offers its own advantages and is suitable for a specific use. For example, Fig. 11 shows the experimental setup for nebulized spray pyrolysis. 80) Nebulized spray pyrolysis (NSP) is a solution-based technique; however, organic solvents can be eliminated as an annealing treatment. The powder size can reach the nanometer scale. Furthermore, the synthesis time is shorter using this NSP technique compared to other techniques. The electrospinning technique was chosen to prepare nanostructured LLZO such as ceramic nanofibers and nanowires. Additionally, the phase transformations can be investigated. However, these methods have not yet matured and cannot meet the large-scale requirements of commercial production. Therefore, researchers are still working on the development of new, efficient, and energy-saving preparation methods that can reduce the cost, sintering temperature, and cycle time while also taking into account the electrochemical performance of LLZO.
4. Element Doping in LLZO
Because of the different diffusion mechanisms, the ionic conductivity of c-LLZO is two orders of magnitude higher than that of t-LLZO. However, the structure of c-LLZO at high temperature is unstable and easily converted into a tetragonal phase. 81,82,113) Therefore, in addition to exploring a reasonable preparation method, stabilizing the cubic phase lattice to improve its ionic conductivity at room temperature is another research focus of LLZO. Element doping is one of the most effective ways to stabilize the cubic phase lattice and improve the ionic conductivity. On the one hand, element doping can regulate the concentration of lithium ions to effectively increase the lithium vacancy concentration and the disorder of the lithium ion arrangement in the lattice, thus stabilizing the cubic phase and optimizing the ionic conductivity. On the other hand, the skeleton structure of LLZO lattice can be adjusted by element doping to increase the lithium ion mobility. As shown in Fig. 12, the transport path of Li + in the LLZO lattice is that Li + migrates from [Li 2O 6] octahedron to an adjacent [LiO 4] tetrahedron, and then migrates to another adjacent [Li 2O 6] octahedron. The bottleneck size of the Li + transport is the size of a triangle composed of three coplanar oxygen atoms of [Li 2O 6] octahedral and [LiO 4] tetrahedral. 83)
Taking the Zr site substitution as an example, because [ZrO 6] octahedrons and [Li 2O 6] octahedrons are co-edge-joined, the difference in the ionic radius between the doping element and Zr can change the bottleneck size of the Li + transport path, which affects the activation energy of lithium ion migration. As a consequence, the bottleneck size of the lithium ion transport has a significant influence on the ionic mobility and ionic conductivity. There are three types of doping and substitution sites for LLZO: Li site, La site, and Zr site. The effect mechanisms of doping at different sites on the ionic conductivity of LLZO are different. The study results of element substitutions for Li, La, and Zr sites in recent years are listed in Table 4.
4.1. Li site substitution
For doping an Li site, researchers usually use Al 3+, Ga 3+, Fe 3+, and other elements to partially substitute the Li element. The substitution of the Li site only affects the lithium ion concentration, and research studies have shown that the concentration and different distributions of lithium ions in LLZO have a crucial influence on its ionic conductivity. Rangasamy et al. 84) found that 0.2-0.24-mol Al substitution in LLZO can stabilize the cubic phase, and their ionic conductivities were higher than that of LLZO without Al doping. This phenomenon was quickly confirmed by other researchers who also studied Al-doped LLZO. 85,86) Subsequently, Bernstein et al. 87) combined density functional theory and molecular dynamics calculations to prove that the introduction of lithium vacancies in a concentration range of 0.4 to 0.5 in the LLZO structural unit is more conducive to the improvement of the ionic conductivity. Jin et al. 88) found that Al doping not only changes the Li + concentration in the sample but also promotes the densification of the sample, resulting in an increase in ionic conductivity. As the Al content increased from 0 to 0.25 wt.%, the density of the sintered sample increased from 2.6 g·cm −3 to 4.5 g·cm −3. Accordingly, the ionic conductivity increased from 1.2 × 10 −7 S/cm to 2.0 × 10 −4 S/cm. Zhang et al. 47) prepared LLZO ceramic pellets with Li + concentration of 5.47 mol to 7.37 mol. The relationship between the Li + concentration and the ionic conductivity was studied by a nuclear magnetic resonance (NMR) test. It was found that when the Li + concentration exceeded 7 mol, the XRD peak of LLZO broadened, indicating that the cubic phase changed to the tetragonal phase. This is the result of a highly ordered arrangement of lithium ions. When the Li + concentration was 6.35 mol, the ionic conductivity reached its peak, indicating that a proper reduction in the Li + concentration (or increase in the lithium vacancy concentration) for LLZO is beneficial to the improvement of ionic conductivity. Therefore, the substitution of nonequivalent cations for Li sites and the use of extra positive charges to promote the formation of lithium vacancies have become research hot spots for improving the ionic conductivity of LLZO. Wolfenstine et al. 89) believed that because of the site preference and slightly larger size of Ga than Al, it is highly possible for Ga-doped LLZO to have a lower occupancy fraction on the Li1 (tetrahedral) site than Al-doped LLZO, thus leading to higher ionic mobility and conductivity. Cubic Li 6.25La 3Zr 2Ga 0.25O 12 powders with a relative density of ~ 91% were synthesized by hot pressing. They had a total Li-ion conductivity of ~3 .5 × 10 −4 S/cm, which was slightly higher than that of Al-doped LLZO of a similar composition and relative density. Wu et al. 90) prepared a series of Li 7-3xGa xLa 3Zr 2O 12 (x = 0.10, 0.15, 0.20, 0.25, 0.30, 0.35, and 0.40) electrolytes via a solid-state reaction. The Li magic angle spinning nuclear magnetic resonance (MAS-NMR) spectra were used to analyze the lithium ion dynamics. Fig. 13(a) shows the X-ray diffraction (XRD) patterns of the Li 7-3xGa xLa 3Zr 2O 12 samples. The patterns and lattice parameters match well with the cubic garnet phase. The conductivity of the Li 7-3xGa xLa 3Zr 2O 12 samples was measured by the AC impedance technique. and to total conductivity is calculated in Table 5.
Figure 13(b) shows the temperature dependence of the total conductivities of the Li 7-3xGa xLa 3Zr 2O 12 samples. It is worth noting that the conductivities of the samples (x ≥ 0.20) with the cubic phase are higher than those of the samples (x < 0.20). A cubic structure is obtained when the Ga concentration exceeds 0.2 per formula unit, while a highest ionic conductivity of 1.46 mS/cm at 25°C was achieved at x = 0.25. A special distribution in which most lithium within LLZO resides at the octahedral 96 h site, away from the central octahedral 48 g site, while the remaining lithium resides at the tetrahedral 24 d site, leads to high lithium-ion mobility as well as high conductivity.
Compared to the doping of Al 3+ and Ga 3+, doping with other elements such as Fe 3+ and Ge 4+ has seldom been studied. Rettenwander et al. 91-93) found a very high total conductivity of 1.1 mS/cm at room temperature, but the Fe-doped LLZO was not stable vs. lithium metal since it formed a thick tetragonal LLZO interlayer, causing high interfacial impedance. 57Fe Mössbauer spectra of Fe xLi 7-3xLa 3Zr 2O 12 is shown in Fig. 14, which indicates that about 96% of the total iron occurs as Fe 3+ and 4% as Fe 2+. Hence, Fe 3+ seems to act similarly as Al 3+ in LLZO which can stabilize the cubic phase of LLZO and promote its ionic conductivity. 91)
As shown in Fig. 15 , the Raman spectroscopy of LLZO/Li interface indicates that there are two different phases of LLZO (cubic phase and tetragonal phase) present in the interfacial layer which causes by the contact of cubic LLZO with Li metal. The formation of t-LLZO will lead to an increased interfacial resistance because of the lower ionic conductivity of t-LLZO. Huang et al. 94) studied the doping effect of In, Si, and Ge. The researchers found that Si and In doping deteriorates the electrical properties of LLZO, while Ge-doped LLZO presents an enhanced ionic conductivity of 7.63 × 10 −4 S/cm at 298 K.
4.2. La site substitution
Thus far, LLZO doping modification is mainly concentrated in the Li and Zr sites because the La site has a greater influence on the entire crystal structure of LLZO and determines the bottleneck size of the lithium ion transport. 120) Therefore, it is difficult to find suitable doping ions for the La site. The supervalent doping of the Li and La sites causes lithium vacancies and disordered lithium sublattices. In contrast to supervalent doping, low-valence doping results in excess lithium ions. It was reported that doping at the La site with a relatively large-radius cation can enhance the conductivity of LLZO. Jiang-Fang Wu et al. 121) doped cation Rb + at the La 3+ site in cubic Li 6.10Ga 0.30La 3Zr 2O 12, which led to a highest ionic conductivity of 1.62 mS/cm at 25°C. The Rb + doping facilitates the densification of the pellet at a lower sintering temperature. Compared with La 3+ (0.106 nm), Rb + has a larger ionic radius of 0.148 nm, which contributes to enlarging the Li + migration pathway and hence increases the ionic conductivity. The preparation method, crystal structure, and Li + migration pathway are shown in Fig. 16. Study 122) showed that the substitution of the La site with an alkaline earth metal can effectively increase the lithium content at the Li 1 site and reduce the lithium content at the Li 2 site in the LLZO structure. The lithium ion at the Li 1 site is easier to migrate than at the Li 2 site. 123, 124) Therefore, alkaline earth metals (e.g., Mg, Ca, and Sr) have a positive effect on the improvement of lithium ion conductivity and the reduction of the grain boundary resistance. It seems suitable to replace La with Sr because the ionic radius of Sr 2+ is very similar to that of La 3+, and Sr can act as a sintering and densification agent. 125) The ionic conductivity of the 1.7 wt.% Sr-doped LLZO at room temperature is up to 5.0 × 10 −4 S/cm by traditional solid-state synthesis, while the conductivity of the undoped LLZO is only 2.1 × 10 −4 S/cm at room temperature. 122) In a recent work, a modified solution method with the influence of elements Ca and Ta doped on LLZO was discussed. 126, 127) The synergetic doping of Ca and Ta was found to induce the formation of cubic LLZO with a high ionic conductivity of 4.03 × 10 −4 S/cm. In addition to the doping of the La site by a monovalent alkali metal and divalent alkaline earth metal, La also can be replaced by a supervalent element such as Ce 4+. Ce 4+ has a similar ionic radius to La 3+ and can appropriately reduce the lithium ion concentration, which is favorable for the improvement of the ionic conductivity. 128) Rangasamy et al. 128) studied the effects of different doping contents (0 < x < 0.8) on the phase and structure of LLZO by the traditional solid-state sintering method. It was found that when the content of Ce 4+ exceeded 0.2, it helped to stabilize the cubic phase lattice structure of LLZO. However, the total ionic conductivity of the Ce-doped LLZO was only 0.014 mS/cm, which was considerably lower than that of the elements substituted by Li and Zr. The lower conductivity was probably caused by CeO 2 precipitation at the grain boundaries, which might lead to a high grain boundary resistance.
4.3. Zr site substitution
Compared to LLZO doping modifications in Li and La sites, Zr site doping modification is the most studied method in research on LLZO. It is a commonly used and effective method to stabilize the lattice of cubic-LLZO by Ta 49,120, 128- 135, 146, 149- 151) doping. In addition, there is extensive research interest in this field, including substituting Zr sites with Ge, 145) Mo, 137) W, 138) Sb, 139, 140) Nb, 141, 142, 152, 153) and Y 143) ions; Nb/Y co-doping (both doping to Zr sites); Ca/Ta co-doping (Ca doping to Li and La sites, Ta doping to Zr sites); Mg/Ta co-doping (Mg doping to Li sites, Ta doping to Zr sites); and Ba/Ta co-doping (Ba doping to Li and La sites, Ta doping to Zr sites). Considering ion conductivity, by far, the doping modification method for Zr sites above will effectively increase the ionic conductivity of electrolyte to the magnitude of 10 −4 S/ cm. Ta doping can easily achieve 10 −3 orders of magnitude among all of these elements. In 2013, Baek 128) processed Ta- LLZO with a high ionic conductivity of 1.35 × 10 −3 S/cm by spark plasma sintering (SPS). In 2014, Sun et al. 132) focused on the density as an impact factor of the ionic conductivity of electrolyte. The researchers fabricated Li 6.4La 3Zr 1.4Ta 0.6O 12 ceramic disks with a 99.6% density by a hot-press sintering technique. This successfully increased the ionic conductivity of Ta-doped LLZO to a magnitude of 1.6 × 10 −3 S/cm. The method of Zr site doping has the potential to reduce the sintering time and temperature. Compared to the solgel method and SPS, when using the traditional method of solid-state sintering, a higher sintering temperature (> 1100°C) and longer sintering time (> 10 h) are necessarily involved in the process. However, Zr site doping contributes to improving the sintering efficiency, which will shorten the process time of traditional solid-state sintering. In addition, energy conservation has a positive impact. W. Xu et al. 136) doped GeO 2 of less than 1 wt.% to fabricate Ge-LLZO with an ionic conductivity up to 8.28 × 10 −4 S/cm at room temperature. This reduced the sintering time and temperature for the solid-state reaction as well. Yu Tang et al. 153) synthesized Nb-LLZO with B 2O 3 by a solid-state reaction method. The cubic LLZN phase was obtained after calcining at 850°C for 6 h. However, the ionic conductivity was relatively low compared to other examples: 1.86 × 10−4 S/cm.
In case of a negative impact on the lattice of LLZO, the choice of doping element will be the determinant. 29) Not only the performance of the doped LLZO, but also the ability to dope depend on the radius of doping cation. Fig. 17 reveals the relationship between the radius of the doped ion and the highest ion conductivity in Zr sites of LLZO in the research. It can be concluded from the results that the electrochemical performance has been improved when the radius of the doped ion lies in the range of 0.5-0.7 Å. In particular, Te doping and Ta doping will enhance the ion conductivity to 10 −3 S/cm. In the meantime, elements with radii that lie in the typical range, such as Ge 4+, Sb 5+, and Nb 5+, will have a positive impact on ionic conductivity. The ionic conductivity will surpass 6 × 10 −4 S/cm after doping these typical elements to Zr sites. It should be noted that all the results above are from the best set of experimental data. Owing to the influence of the sintering process on the material structure and different doping concentrations, the results of the ionic conductivity will turn out to be different even when the same element has been used for doping. For example, the ionic conductivity may still be as low as 2 × 10 −4 S/cm 146) with single doping, even when doping with the most commonly used element Ta. The disappointing performance is caused by factors such as low density. Co-doping and single-element doping have attracted extensive research interest. For example, Xiaolan Chen et al. 148) co-doped Ca and Ta to LLZO (Ca in the Li and La sites, Ta in the Zr sites). The synergetic effect from Ta and Ca enhanced the ion conductivity to a relatively high magnitude of 2.84 × 10 −4 S/cm compared to that of single Ta-doped LLZO (1.95 × 10 −4 S/cm). The reason is that Ta plays a key role in stabilizing the cubic phase, and a small amount of Ca compensates the loss of Li content caused by doping a pentavalent Ta ion. However, although co-doping will stabilize the lattice of cubic LLZO, sometimes its ion conductivity may be lower than the single-element doped LLZO of these elements; this aspect needs to be addressed. Xiao Huang et al. 156) prepared Ta-LLZO with MgO, which led to a 5.0 × 10 −4 S/cm ion conductivity at room temperature. Unfortunately, pure LLZTO has a higher ion conductivity of 7.3 × 10 −4 S/cm compared to the result of the above co-doping. At present, the progress of experimental research on Zr site doping has slowed, while the theoretical calculation is still in progress. At the same time, the study of single doping is basically complete for those elements that have the potential to improve or affect the performance of LLZO electrolyte. While the mechanism of double doping or even multiple- element co-doping is more complicated, research on theoretical analysis and explanation is lacking. This has delayed the experimental study of this topic. As with other sites, Zr site doping can lead to the lattice distortion of the materials, so the size of the lithium ion transmission channel is affected which in turn changes the ionic conductivity of LLZO. In addition to focusing on simple element replacement, it is necessary to study the Zr site doping from more angles and deeper levels, and this is also the future research trend.
5. Conclusions
Garnet-type inorganic solid electrolyte Li7La3Zr2O12 (LLZO) possesses high ionic conductivity (10−4-10−3 S/cm) at room temperature and low electronic conductivity (< 10−11 S/cm), as well as the advantages of good chemical stability against lithium anodes, good thermal stability, and a wide electrochemical window, etc. Thus, it has significant research value and wide practical application prospects in the development of all-solid-state lithium batteries. In recent years, extensive research studies have been conducted on the Liion transport mechanism, element doping, and preparation methods of LLZO in order to improve the ionic conductivity while reducing the manufacturing cost, decreasing the sintering temperature, and shortening the synthesis cycle.
However, to realize the commercialization and practical application of all-solid-state lithium batteries, there are still some issues to be solved. First, LLZO is reactive with atmospheric H2O and CO2 even at room temperature, which deteriorates the ionic conductivity. Thus, the production process is preferably taken under an inert atmosphere, or additives can be used to suppress the unfavorable reaction. Then, the inhibition and mechanism of the lithium dendrite problem need further investigation. Finally, the large interfacial resistance and unstable solid-solid interface contact between LLZO solid electrolyte and electrodes caused by its intrinsic high rigidity are the main problem in its practical application because these strongly affect the cycle stability and rate capability of the batteries. Therefore, the current research hot spot has gradually shifted to interface issues, which are key to the industrial production and practical application of LLZO.
Acknowledgments
This work was financially supported by the National Key Research and Development Program of China (No. 2018YFB0905600 and 2017YFB0310400), the National Natural Science Foundation of China (No. 51472188 and 51521001), Fundamental Research Funds for the Central Universities in China, State Key Laboratory of Advanced Electromagnetic Engineering and Technology (Huazhong University of Science and Technology), and the “111” project (No. B13035).
Fig. 1
Crystal structure of (a) tetragonal LLZO, (b) transformed tetragonal LLZO, and (c) cubic LLZO. Lithium arrangement in (d) tetragonal and (e) cubic LLZO. 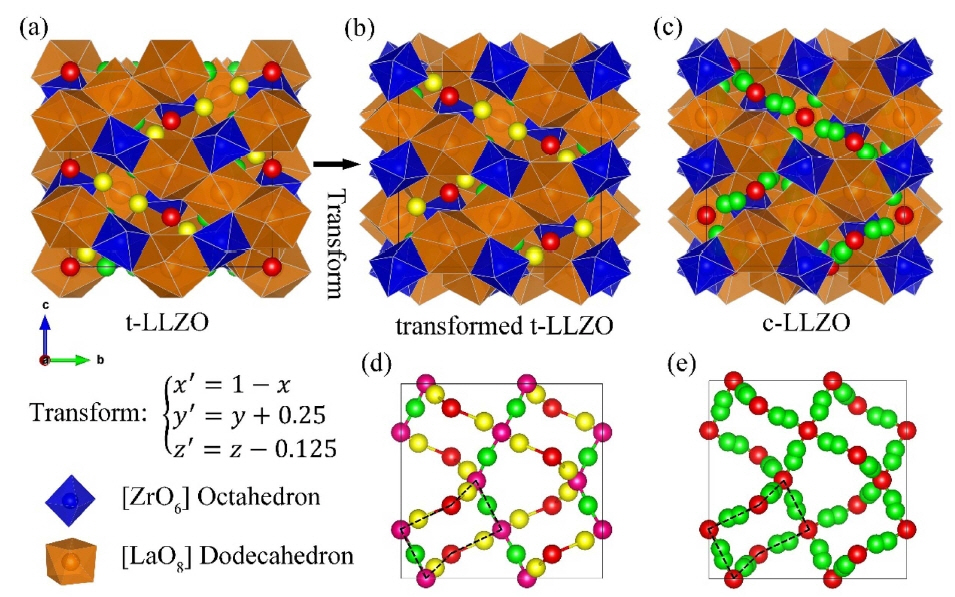
Fig. 2
Loop structure of lithium arrangement in (a) cubic and (b) tetragonal LLZO. g represents occupancy of each site. 31)
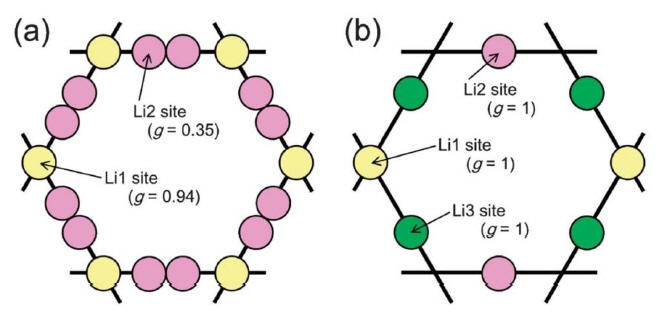
Fig. 3
(a) In situ XRD pattern of LLZO in temperature range of 303 to 973 K. Temperature-dependent lattice parameters of LLZO from (b) Rietveld refinement results and (c) molecular dynamic simulation. 40)
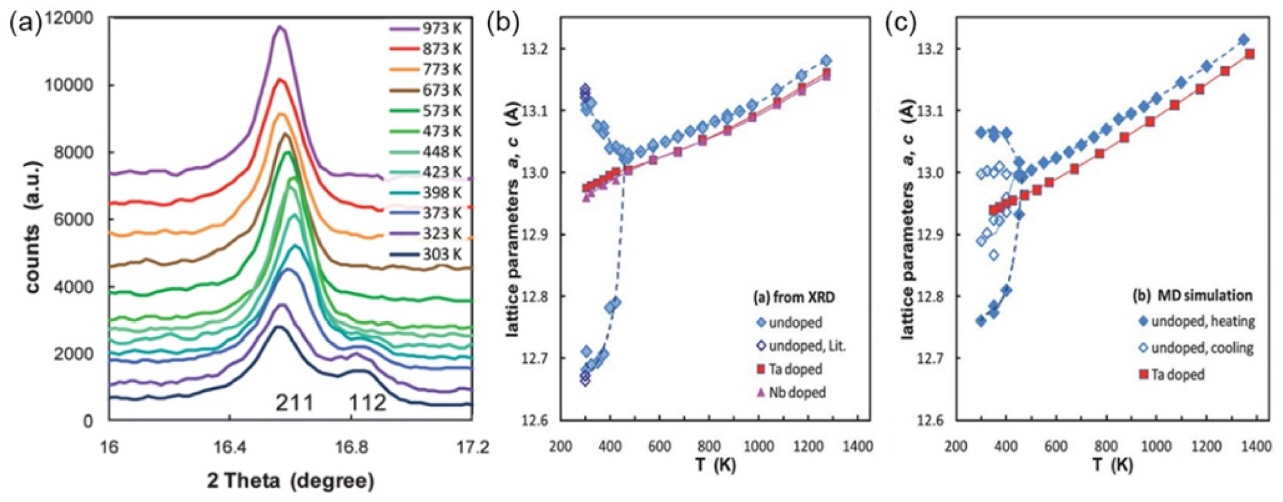
Fig. 4
(a) Cage occupancy for four types of lithium sites as function of temperature. (b) Mean square displacement of lithium atoms for different cages. 38)
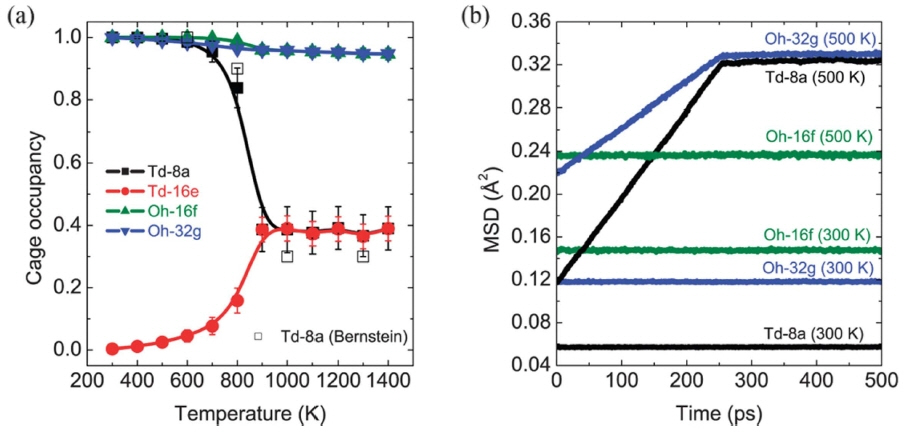
Fig. 5
Root mean square deviation for individual lithium ions of (a) tetragonal and (b) cubic LLZO at 300 K. 42)
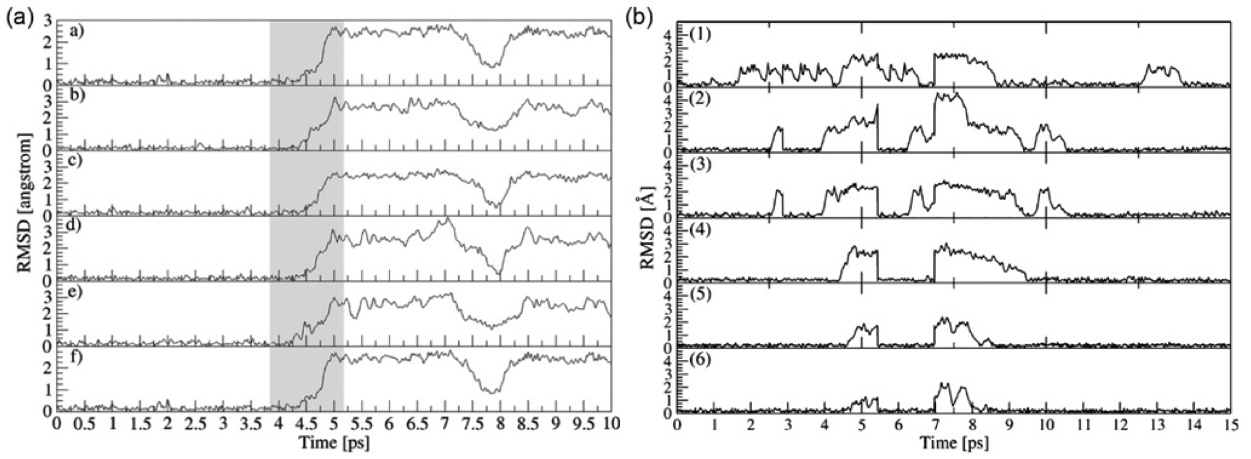
Fig. 6
Li hopping statistics from molecular dynamic simulation compared to ideal Poisson process. k is jump event number per unit cell in 1 ps. 44)
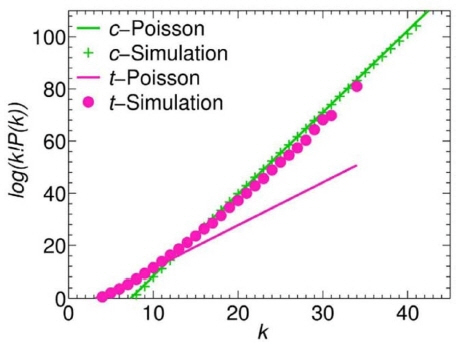
Fig. 7
XRD patterns of Al-doped LLZO samples (▼ LaAlO 3 and ▽ La 2Zr 2O 7). 52)
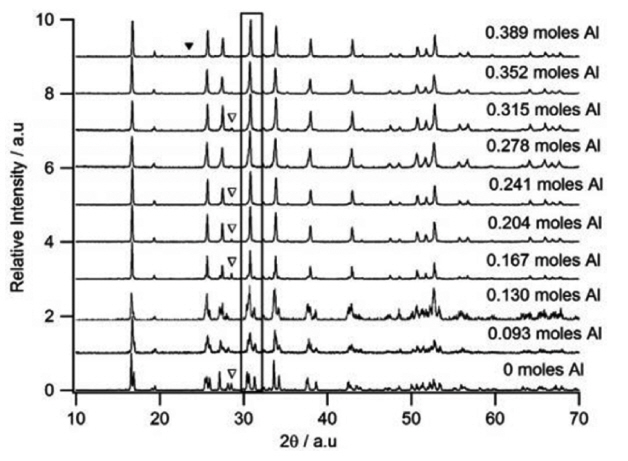
Fig. 8
SEM micrographs of LLZO powder precursors calcined at (a) 923 K, (b) 973 K, (c) 1073 K, and (d) 1173 K in air atmosphere for 5 h. 70)
Fig. 9
Schematic diagram of FAST. 
Fig. 10
SEM micrographs of cross sections of LLZO samples sintered by FAST at (a) 1150°C and (b) 1200°C. 45)
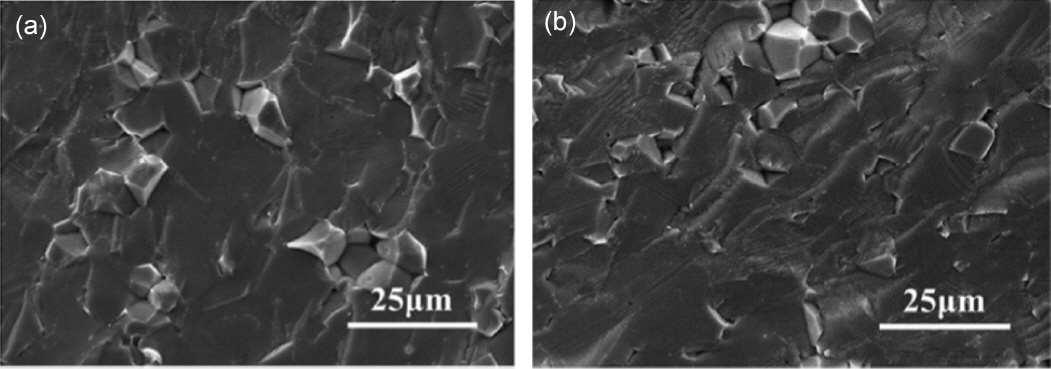
Fig. 11
Experimental setup for nebulized spray pyrolysis. 80)
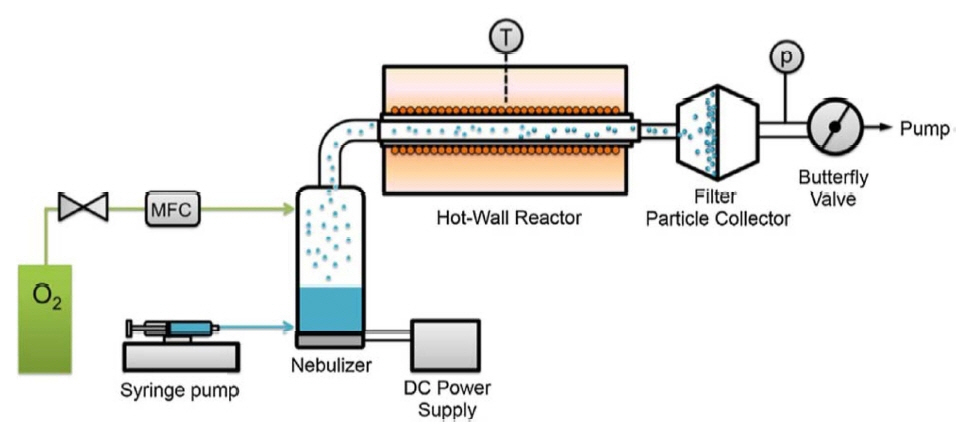
Fig. 12
(a) Li migration path and (b) bottleneck size of lithium- ion transportation path. 83)

Fig. 13
(a) XRD patterns of Li 7-3xGa xLa 3Zr 2O 12 samples and (b) temperature dependence of conductivity of Li 7-3xGa xLa 3Zr 2O 12 samples. 90)
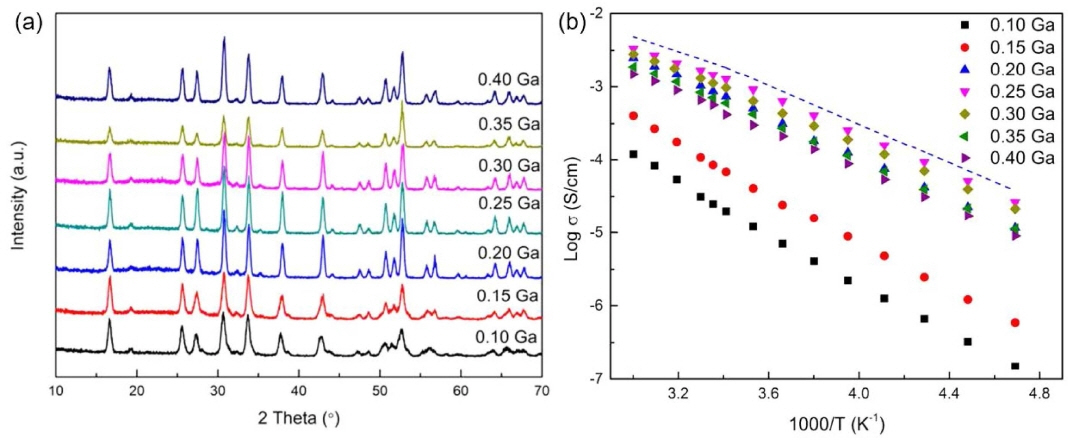
Fig. 14
57Fe Mössbauer spectra of Fe xLi 7-3xLa 3Zr 2O 12 at (a) 295 K and (b) 80 K. Both spectra were evaluated using 4 quadrupole splitting distributions. 91)

Fig. 15
(a) Raman mapping of the Li 6.4Fe 0.2La 3Zr 2O 12; the picture shows a cross section after the solid electrolyte has been in contact with metallic lithium during cyclic voltammetry. (b) Representative spectra of the different areas of the cross section. (c) Magnification of the shaded area in (b) showing cubic and tetragonal LLZO. Color code: Cubic LLZO (blue), tetragonal LLZO (red), (distorted spectrum of) tetragonal LLZO (green), diamond (yellow), epoxy resin (orange). 93)
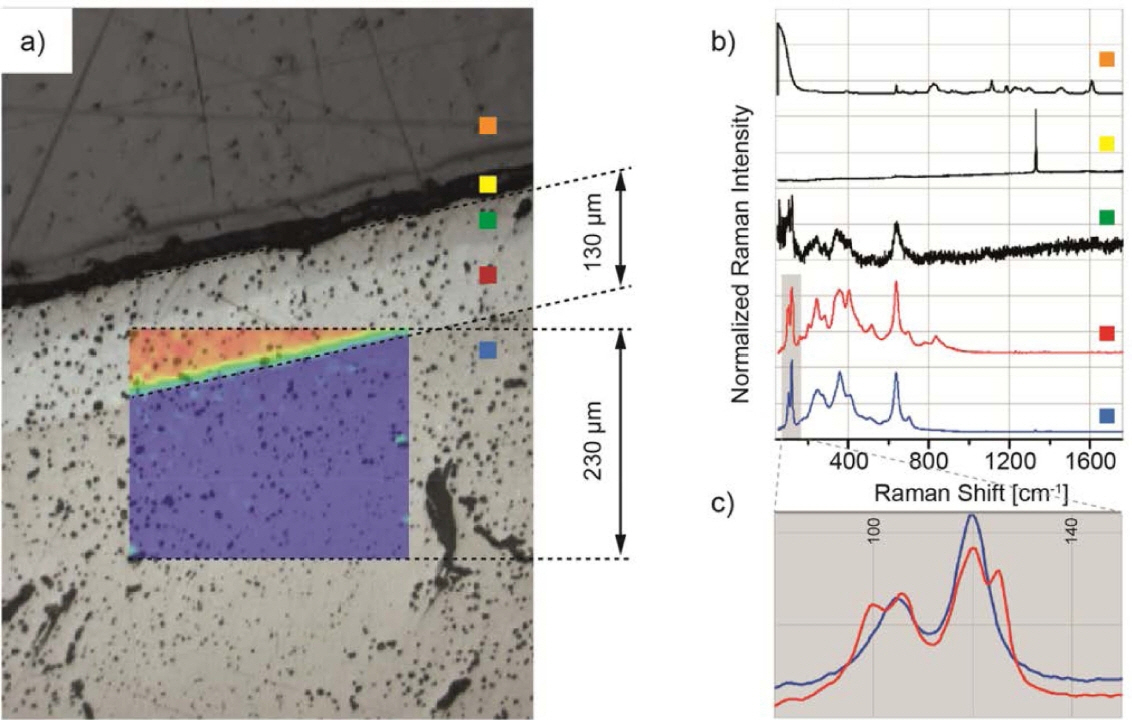
Fig. 16
(a) Preparation process of electrolytes, (b) optical image, (c) crystal structure displayed along [100] direction for Li 6.10+2yGa 0.30La 3-yRb yZr 2O 12 garnet-type solid, (d) schematic of bottleneck in Li + migration pathway, and (e) schematic of two migration pathways for Li +. 121)

Fig. 17
Summary of dopant ion radiation and ion conductivity of doped LLZO (red circle represents undoped LLZO, purple circles represent doped LLZO). 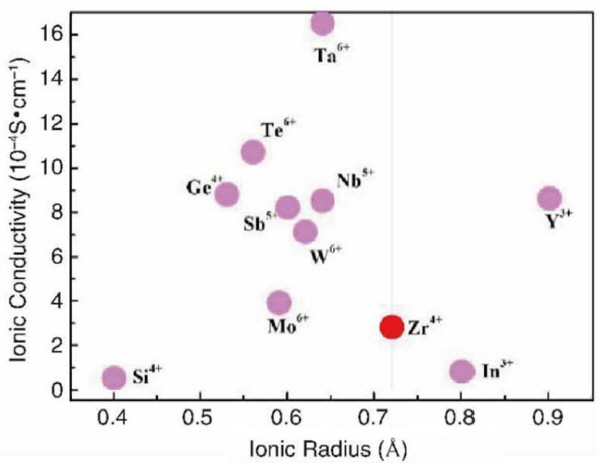
Table 1
Preparation of Cubic LLZO by Conventional Solid-statee Reaction
|
Chemical formula |
Year |
Li+ conductivity at RT (×10−3 S/cm) |
Relative density |
Technology |
Ea (eV) |
Ref. |
|
Li7La3Zr2O12
|
2007 |
0.30 |
/ |
1230°C, 36 h |
0.32 |
[30] |
|
Li6.75La3Zr1.75Nb0.25O12
|
2010 |
0.80 |
/ |
1200°C, 36 h |
0.31 |
[60] |
|
Li7.06La3Y0.06Zr1.94O12
|
2011 |
0.81 |
/ |
1200°C, 16 h |
0.26 |
[61] |
|
Li6.4La3Zr1.75Ta0.6O12
|
2012 |
1.0 |
/ |
1140°C, 16 h |
0.35 |
[56] |
|
Li6.4La3Zr1.6W0.3O12
|
2013 |
0.79 |
/ |
1100°C, 36 h |
0.45 |
[62] |
|
Li6.6La3Zr1.6Sb0.4O12
|
2013 |
0.77 |
91% |
1100°C, 24 h |
0.34 |
[63] |
|
Li6.75La3Zr1.875Te0.125O12
|
2013 |
0.33 (30°C) |
/ |
1100°C, 15 h |
0.41 |
[64] |
|
Li6.28Al0.24La3Zr2O12
|
2015 |
0.44 (30°C) |
93% |
1200°C, 12 h |
0.37 |
[65] |
|
Li5.9Al0.2La3Zr1.75W0.25O12
|
2015 |
0.49 |
/ |
1150°C, 12 h |
0.35 |
[66] |
|
Li6.25Ga0.25La3Zr2O12
|
2016 |
1.46 |
94.1% |
1100°C, 24 h |
0.25 |
[58] |
|
Li6.4Fe0.2La3Zr2O12
|
2018 |
1.1 |
/ |
1230°C, 6 h |
/ |
[67] |
|
Li7La3ZrNb0.5Y0.5O12
|
2018 |
0.83 (30°C) |
90.7% |
1230°C, 15 h |
0.31 |
[57] |
Table 2
Preparation of Cubic LLZO by Sol-Gel Method
|
Chemical formula |
Li+ conductivity at RT (×10−4 S/cm) |
Year |
Technology |
Ea (eV) |
Ref. |
|
Li7La3Zr2O12
|
0.0031 |
2011 |
900°C, 5 h |
0.67 |
[70] |
|
Li6La3Zr2O11.5
|
0.14 |
2011 |
1180°C, 32 h |
/ |
[72] |
|
Al-Li7La3Zr2O12
|
0.20 |
2011 |
1200°C, 6 h |
/ |
[73] |
|
Al-Li7La3Zr2O12
|
0.14 |
2012 |
1200°C, 36 h |
0.34 |
[74] |
|
Li7La3Zr2O12
|
0.40 |
2013 |
1000°C, 4 h |
0.41 |
[71] |
|
Li7La3Zr1.89Al0.15O12
|
0.34 |
2013 |
1150°C, 1 h |
0.33 |
[75] |
|
Al-Li7La3Zr2O12
|
0.019 |
2014 |
900°C, 5 h |
/ |
[76] |
|
Li6.42Al0.32La3Zr1.91O12.02
|
0.015 |
2015 |
1000°C, 7 h |
/ |
[77] |
|
Li7La3Zr2O12
|
0.14 |
2016 |
1100°C, 5 h |
/ |
[78] |
Table 3
Comparison of Three Synthesis Methods 45)
|
Synthesis technique |
Technology |
Density (%) |
Li+ conductivity at RT (×10−4 S/cm) |
|
conventional solid-state reaction |
1230°C, 36 h |
96.0 |
3.6 |
|
sol-gel method |
1200°C, 16 h |
86.3 |
2.0 |
|
FAST |
1150°C, 10 min |
99.8 |
5.7 |
Table 4
Composition, Preparation, and Ionic Conductivity of LLZO
|
Composition |
Technology |
Method |
Li+ Conductivity (S/cm) |
Test Temperature |
Reference |
|
Al-LLZO |
1150°C/3 min |
Field assisted sintering technology |
5.7 × 10−4
|
RT |
[45] |
|
Al-LLZO |
1230°C/12 h |
conventional solid-state method |
3.4 × 10−4
|
RT |
[95] |
|
Li6.64Al0.12La3Zr2O12
|
1100°C/3 h |
hybrid sol-gel solid-state procedure |
3 × 10−4
|
RT |
[96] |
|
Li6.25Al0.25La3Zr2O12
|
1100°C/1 h |
conventional solid-state method |
|
|
[97] |
|
Al-LLZO |
1100°C/12 h |
conventional solid-state method |
1 × 10−4
|
|
[98] |
|
Li6.16Al0.28La3Zr2O12
|
900°C/12 h |
modified sol-gel method |
1.92 × 10−5
|
RT |
[99] |
|
Li6.25Al0.25La3Zr2O12
|
850°C |
co-precipitation technique |
|
|
[100] |
|
Li6.25Al0.25La3Zr2O12
|
700°C/6 h |
modified sol-gel process |
9.9 × 10−5
|
25°C |
[101] |
|
Li6.4Al0.2La3Zr2O12
|
1150°C/0.5 h |
microwave sintering method |
1.15 × 10−4
|
RT |
[102] |
|
Li5.92Al0.36La3Zr2O12
|
1150°C/10 h |
three step solid-state reaction |
2.45 × 10−4
|
RT |
[103] |
|
Li6.61La3Zr2Al0.13O11.98
|
1100°C/3 h |
two-step synthesis |
1.35 × 10−4
|
RT |
[104] |
|
Li6.4Al0.2La3Zr2O12
|
1210°C/15 h |
conventional solid-state method |
2.10 × 10−4
|
25°C |
[105] |
|
Li6.23Al0.26La3Zr1.88O11.76
|
1150°C/6 h |
solid-liquid composite method |
2.54 × 10−4
|
23°C |
[106] |
|
Li6.25Al0.25La3Zr2O12
|
1100°C/1 h |
rapid-induction hot-pressing technique |
|
|
[107] |
|
Li6.7Al0.1La3Zr2O12
|
1150°C/15 h |
conventional solid-state method |
1.41 × 10−4
|
30°C |
[108] |
|
Li6.43Fe0.19La3Zr2O12
|
1100°C/17 h |
high-temperature sintering method |
|
|
[91] |
|
Li6.54Fe0.12La3.01Zr1.98O12
|
1050°C/16 h |
high-temperature sintering method |
|
|
[92] |
|
Li6.4Fe0.2La3Zr2O12
|
1230°C/6 h |
high-temperature sintering method |
1. 1 × 10−3
|
RT |
[93] |
|
Li6.55Ga0.15La3Zr2O12
|
1085°C/6 h |
citric acid-nitrate route |
1.3 × 10−3
|
24°C |
[109] |
|
Li6.25La3Zr2Ga0.25O12
|
1000°C/4 h |
hot-pressing method |
3.5 × 10−4
|
RT |
[110] |
|
Li7−3xGaxLa3Zr2O12(x=0.1-0.6) |
1230°C/6 h |
conventional solid-state method |
|
|
[111] |
|
Ga-LLZO |
900°C/2 h |
Couette-Taylor reactor |
1.2-1.75 × 10−3
|
25°C |
[112] |
|
Li5.5La3Zr2Ga0.5O12
|
1100°C/5 h |
sol-gel process |
5.81 × 10−5
|
RT |
[113] |
|
Li6.25Ga0.25La3Zr2O12
|
1100°C/24 h |
solid-state reaction |
1.46 × 10−3
|
25°C |
[90] |
|
Li6.55Ga0.15La3Zr2O12
|
1150°C/6 h |
chelate-gel route |
|
|
[114] |
|
Li6.55Ga0.15La3Zr2O12
|
1150°C/6 h |
sol-gel method |
8.2 × 10−4
|
25°C |
[115] |
|
Li6.46Ga0.18La3Zr2O12
|
700°C/2 h |
radio-frequency magnetron co-sputtering approach |
1.6 × 10−5
|
30°C |
[116] |
|
Li6.55Ga0.15La3Zr2O12
|
1160°C/2 h |
hot-isostatic pressing |
1.13 × 10−3
|
25°C |
[117] |
|
Li6.55Ga0.15La3Zr2O12
|
1075°C/12 h |
solid-state reaction technique |
2.06 × 10−3
|
25°C |
[118] |
|
Li7Al0.2−xGaxLa3Zr2O12
|
1230°C/4 h |
high-temperature sintering route |
0.3-1.32 × 10−3
|
|
[119] |
|
Li6.20Ga0.30La2.95Rb0.05Zr2O12
|
1100°C/4 h |
solid-state reaction |
1.62 × 10−3
|
RT |
[121] |
|
Sr (1.7 wt.%) -LLZO |
1200°C/24 h |
Conventional solid-state method |
5.0 × 10−4
|
24°C |
[122] |
|
Li6.6La2.6Ce0.4Zr2O12
|
1050°C/1 h |
hot-pressing method |
1.4 × 10−5
|
|
[128] |
|
Li6.45Ca0.05La2.95Ta0.6Zr1.4O12
|
1125°C/6 h |
Modified solution method |
4.03 × 10−4
|
|
[126] |
|
Li7Al0.1La3Zr1.75Nb0.25O12
|
1000°C/6 h |
solid-state reaction |
2.83 × 10−4
|
|
[129] |
|
Li6.775Al0.05La3Zr1.925Sb0.075O12
|
1170°C/6 h |
conventional solid-state reaction |
4.10 × 10−4
|
30°C |
[130] |
|
Ta-LLZO |
1000°C/4 h |
conventional solid-state method |
1.35 × 10−3
|
|
[128] |
|
Li7−xLa3Zr1.5Ta0.5O12−d
|
1100°C |
SPS |
1.6 × 10−3
|
80°C |
[131] |
|
Li6.4La3Zr1.4Ta0.6O12
|
950°C |
conventional solid-state method |
2.3 × 10−4
|
80°C |
[132] |
|
Li6.4La3Zr1.4Ta0.6O12
|
950°C |
|
3.7 × 10−4
|
33°C |
[133] |
|
Li6.625La3Zr1.625Ta0.375O12
|
1000°C/12 h |
high temperature ceramic processing |
5 × 10−4
|
RT |
[134] |
|
Li6.6La3Zr1.6Ta0.4O12
|
950°C/5 h |
solid-state sintering method |
3.7 × 10−4
|
RT |
[135] |
|
Ge-LLZO |
1200°C/20 h |
conventional solid-state method |
8.28 × 10−4
|
RT |
[136] |
|
Mo-LLZO |
1230°C |
|
3.4 × 10−4
|
RT |
[137] |
|
LLZWO |
1200°C/12 h |
conventional solid-state method |
6.6 × 10−4
|
|
[138] |
|
Li6.6La3Zr1.6Sb0.4O12
|
1100°C |
conventional solid-state method |
7.7 × 10−4
|
RT |
[139] |
|
Li7−xLa3Zr2−xSbxO12
|
1160°C |
conventional solid-state method |
3.4 × 10−4
|
|
[140] |
|
Li7−XLa3(Zr2−X,NbX)O12
|
1200°C |
conventional solid-state method |
8.0 × 10−4
|
25°C |
[141] |
|
Li6.75La3Nb0.25Zr1.75O12
|
950°C/12 h |
conventional solid-state method |
|
|
[142] |
|
Y-LLZO |
1200°C/16 h |
conventional solid-state method |
8.1 × 10−4
|
25°C |
[143] |
|
Li7La3ZrNb0.5Y0.5O12
|
1230°C/15 h |
conventional solid-state method |
8.29 × 10−4
|
30°C |
[144] |
|
Li7La3Zr1.7Ge0.3O12
|
1160°C/12 h |
conventional solid-state method |
4.78 × 10−4
|
20°C |
[145] |
|
LixLa3Zr1.5 Ta0.5 O12
|
1100°C/4 h |
sol-gel |
2.1 × 10−4
|
|
[146] |
|
Li7.2La3Zr1.8Gd0.2O12
|
1220°C/36 h |
conventional solid-state method |
2.3 × 10−4
|
RT |
[147] |
|
Li6.45Ca0.05La2.95Ta0.6Zr1.4O12
|
1125°C/6 h |
conventional solid-state method |
2.84 × 10−4
|
|
[148] |
|
Li6.4La3Zr1.4Ta0.6O12
|
900°C/6 h |
simple solution casting |
4.8 × 10−4
|
60°C |
[149] |
|
Li6.75La3Zr1.75Ta0.25O12
|
900°C/6 h + 1100°C/4 h |
conventional solid-state method |
10−3
|
RT |
[150] |
|
Li5.28La2.92Ta1.09Zr0.91O12
|
1100°C/16 h |
conventional Czochralski technique |
10−3
|
RT |
[151] |
|
Li6.3La2.9Ca0.2Zr1.4Ta0.6O12
|
900°C/2 h |
sol-gel |
2.0 × 10−4
|
RT |
[152] |
|
Li6.5La3Zr1.5Nb0.5O12
|
850°C/6 h |
conventional solid-state method |
1.86 × 10−4
|
|
[153] |
|
Li6.375La3Zr1.375Nb0.625O12
|
|
|
1.37 × 10−3
|
RT |
[154] |
|
Li6.5La2.5Ba0.5ZrTaO12
|
1100°C/12 h |
conventional solid-state method |
1.5 × 10−4
|
25°C |
[155] |
|
Li6.4La3Zr1.4Ta0.6O12-MgO composite |
1250°C/80 min + 1150°C/5 h |
conventional solid-state method |
5.0 × 10−4
|
RT |
[156] |
Table 5
Chemical Composition, Relative Density, Total Conductivity, Activation Energy, and Electronic Conductivity of Li 7−3xGa xLa 3Zr 2O 12 Samples 90)
|
x |
Li:La:Zr:Ga |
Relative density (%) |
σtotal (mS/cm) |
Ea (eV) |
σelectronic (10−7 S/cm) |
|
|
|
Nominal |
ICP-OES resulta
|
Overall |
High T
|
Low T
|
|
0.10 |
6.70:3:2:0.10 |
|
86.7 |
0.025 |
0.34 |
|
|
|
|
0.15 |
6.55:3:2:0.15 |
|
89.6 |
0.085 |
0.33 |
|
|
|
|
0.20 |
6.30:3:2:0.20 |
7.02:3:1.95:0.19 |
93.4 |
0.87 |
0.28 |
0.26 |
0.28 |
1.4 |
|
0.25 |
6.25:3:2:0.25 |
6.79:3:1.97:0.25 |
94.1 |
1.46 |
0.25 |
0.20 |
0.26 |
0.54 |
|
0.30 |
6.10:3:2:0.30 |
6.32:3:1.96:0.29 |
96.3 |
1.12 |
0.25 |
0.23 |
0.25 |
0.47 |
|
0.35 |
5.95:3:2:0.35 |
6.43:3:1.99:0.33 |
95.1 |
0.71 |
0.26 |
0.24 |
0.27 |
0.81 |
|
0.40 |
5.80:3:2:0.40 |
6.64:3:2.02:0.42 |
92.8 |
0.57 |
0.26 |
0.26 |
0.26 |
1.1 |
REFERENCES
1. RP. Luo, WQ. Lyu, and KC. Wen, “Overview of Graphene as Anode in Lithium-Ion Batteries,” J Electron Sci Technol, 16 [1] 57-68 (2018).
2. JB. Goodenough, and KS. Park, “The Li-Ion Rechargeable Battery: A Perspective,” J Am Chem Soc, 135 [4] 1167-76 (2013).  3. JB. Bates, NJ. Dudney, and BJ. Neudecker, “Thin-Film Lithium and Lithium-Ion Batteries,” Solid State Ionics, 135 33-45 (2000).  4. JM. Tarascon, and M. Armand, “Issues and Challenges Facing Rechargeable Lithium Batteries,” Nature, 414 359-67 (2001).  5. C. Sun, J. Liu, and Y. Gong, “Recent Advances in All-Solid-State Rechargeable Lithium Batteries,” Nano Energy, 33 363-86 (2017).  6. C. Cao, Z. Li, and XL. Wang, “Recent Advances in Inorganic Solid Electrolytes for Lithium Batteries,” Front Energy Res, 2 25(2014).  7. F. Croce, GB. Appetecchi, L. Persi, and B. Scrosati, “Nanocomposite Polymer Electrolytes for Lithium Batteries,” Nature, 394 456-58 (1998).   8. H. Aono, N. Imanaka, and GY. Adachi, “High Li+ Conducting Ceramics,” Acc Chem Res, 27 [9] 265-70 (1994).  9. P. Knauth, “Inorganic Solid Li Ion Conductors: An Overview,” Solid State Ionics, 180 [14-16] 911-16 (2009).  10. K. Arbi, MG. Lazarraga, DBH. Chehimi, M. Ayadi-Trabelsi, JM. Rojo, and J. Sanz, “Lithium Mobility in Li1.2Ti1.8R0.2 (PO4)3 Compounds (R = Al, Ga, Sc, In) as Followed by NMR and Impedance Spectroscopy,” Chem Mater, 16 [2] 255-62 (2004).  11. OI. V’yunov, ON. Gavrilenko, and LL. Kovalenko, “Intercalation Processes Influence the Structure and Electrophysical Properties of Lithium-Conducting Compounds Having Defect Perovskite Structure,” Russ J Inorg Chem, 56 [1] 93-8 (2011).  12. Y. Inaguma, and M. Nakashkima, “A Rechargeable Lithium-Air Battery Using a Lithium Ion - Conducting Lanthanum Lithium Titanate Ceramics as an Electrolyte Separator,” J Power Sources, 228 250-55 (2013).  13. A. Mei, XL. Wang, and JL. Lan, “Role of Amorphous Boundary Layer in Enhancing Ionic Conductivity of Lithium-Lanthanum Titanate Electrolyte,” Electrochim Acta, 55 [8] 2958-63 (2010).  14. X. Guo, PS. Maram, and A. Navrotsky, “A Correlation between Formation Enthalpy and Ionic Conductivity in Perovskite-Structured Li3xLa0.67-xTiO3 Solid Lithium Ion Conductors,” J Mater Chem A, 5 [25] 12951-57 (2017).  15. R. Qin, Y. Wei, and T. Zhai, “LISICON Structured Li3V2(PO4)3 with High Rate and Ultralong Life for Low-Temperature Lithium-Ion Batteries,” J Mater Chem A, 6 [20] 9737-46 (2018).  16. V. Thangadurai, and W. Weppner, “Recent Progress in Solid Oxide and Lithium Ion Conducting Electrolytes Research,” Ionics, 12 [1] 81-92 (2006).  17. Y. Su, J. Falgenhauer, and A. Polity, “LiPON Thin Films with High Nitrogen Content for Application in Lithium Batteries and Electrochromic Devices Prepared by RF Magnetron Sputtering,” Solid State Ionics, 282 63-9 (2015).  18. J. Jürgen, and WG. Zeier, “A Solid Future for Battery Development,” Nat Energy, 1 [9] 16141(2016).   19. Y. Kato, S. Hori, and T. Saito, “High-Power All-Solid-State Batteries Using Sulfide Superionic Conductors,” Nat Energy, 1 16030(2016).  20. J. Saienga, and SW. Martin, “The Comparative Structure, Properties, and Ionic Conductivity of LiI+ Li2S+ GeS2 Glasses Doped with Ga2S3 and La2S3
,” J Non-Cryst Solids, 354 [14] 1475-86 (2008).  21. P. Zhao, Y. Wen, and J. Cheng, “A Novel Method for Preparation of High Dense Tetragonal Li7La3Zr2O12
,” J Power Sources, 344 56-61 (2017).  22. R. Murugan, V. Thangadurai, and W. Weppner, “Fast Lithium Ion Conduction in Garnet-Type Li7La3Zr2O12
,” Angew Chem, Int Ed, 46 [41] 7778-81 (2007).  23. X. Han, Y. Gong, and K. Fu, “Negating Interracial Impedance in Garnet-Based Solid-State Li Metal Batteries,” Nat Mater, 16 [5] 572-79 (2016).   24. M. Abreu-Sepúlveda, “Synthesis and Characterization of Substituted Garnet and Perovskite-Based Lithium-Ion Conducting Solid Electrolytes,” Ionics, 22 [3] 317-25 (2016).   25. C. Deviannapoorani, LS. Shankar, and S. Ramakumar, “Investigation on Lithium Ion Conductivity and Structural Stability of Yttrium-Substituted Li7La3Zr2O12
,” Ionics, 22 [8] 1281-89 (2016).   26. Y. Li, “W-Doped Li7La3Zr2O12 Ceramic Electrolytes for Solid State Li-Ion Batteries,” Electrochim Acta, 180 [1] 37-42 (2015).  27. J. Li, Y. Jiang, and H. Zhou, “Effects of Sintering Aids Al2O3 and Y2O3 on the Lithium Ion Conductivity of Solid Lithium Ion Electrolyte LLZO,” Mater Sci Eng Powder Metal, 23 [2] 199-205 (2018).  28. S. Yu, and DJ. Siegel, “Grain Boundary Contributions to Li-ion Transport in the Solid Electrolyte Li7La3Zr2O12 (LLZO),” Chem Mater, 29 [22] 9639-47 (2018).  29. V. Thangadurai, H. Kaack, and WJF. Weppner, “Novel Fast Lithium Ion Conduction in Garnet-Type Li5La3M2O12 (M = Nb, Ta),” J Am Ceram Soc, 86 [3] 437-40 (2003).  30. R. Murugan, V. Thangadurai, and W. Weppner, “Fast Lithium Ion Conduction in Garnet-Type Li7La3Zr2O12
,” Angew Chem, Int Ed, 46 [41] 7778-81 (2007).  31. J. Awaka, A. Takashima, K. Kataoka, N. Kijima, Y. Idemoto, and J. Akimoto, “Crystal Structure of Fast Lithium-Ion-Conducting Cubic Li7La3Zr2O12
,” Chem Lett, 40 [1] 60-2 (2011).  32. MP. O’Callaghan, and EJ. Cussen, “The Structure of the Lithium-Rich Garnets Li6SrLa2M2O12 and Li6.4Sr1.4La1.6M2O12 (M = Sb, Ta),” Solid State Sci, 10 [4] 390-95 (2008).  33. J. Awaka, N. Kijima, H. Hayakawa, and J. Akimoto, “Synthesis and Structure Analysis of Tetragonal Li7La3Zr2O12 with the Garnet-Related Type Structure,” J Solid State Chem, 182 [8] 2046-52 (2009).  34. J. Percival, E. Kendrick, RI. Smith, and PR. Slater, “Cation Ordering in Li Containing Garnets: Synthesis and Structural Characterisation of the Tetragonal System, Li7La3Sn2O12
,” Dalton Transactions, [26] 5177-81 (2009).  35. J. Awaka, N. Kijima, K. Kataoka, H. Hayakawa, K. Ohshima, and J. Akimoto, “Neutron Powder Diffraction Study of Tetragonal Li7La3Hf2O12 with the Garnet-Related Type Structure,” J Solid State Chem, 183 [1] 180-85 (2010).  36. M. Matsui, K. Sakamoto, K. Takahashi, A. Hirano, Y. Takeda, O. Yamamoto, and N. Imanishi, “Phase Transformation of the Garnet Structured Lithium Ion Conductor: Li7La3Zr2O12
,” Solid State Ionics, 262 155-59 (2014).  37. N. Bernstein, MD. Johannes, and K. Hoang, “Origin of the Structural Phase Transition in Li7La3Zr2O12
,” Phys Rev Lett, 109 [20] 1-5 (2012).  38. M. Klenk, and W. Lai, “Local Structure and Dynamics of Lithium Garnet Ionic Conductors: Tetragonal and Cubic Li7La3Zr2O12
,” Phys Chem Chem Phys, 17 [14] 8758-68 (2015).  39. MJ. Klenk, and W. Lai, “Finite-Size Effects on the Molecular Dynamics Simulation of Fast-Ion Conductors: A Case Study of Lithium Garnet Oxide Li7La3Zr2O12
,” Solid State Ionics, 289 143-49 (2016).  40. S. Adams, and RP. Rao, “Ion Transport and Phase Transition in Li7-xLa3(Zr2-xMx)O12 (M = Ta5+, Nb5+, x = 0, 0.25),” J Mater Chem, 22 [4] 1426-34 (2012).  41. F. Chen, J. Li, Z. Huang, Y. Yang, Q. Shen, and L. Zhang, “Origin of the Phase Transition in Lithium Garnets,” J Phys Chem C, 122 [4] 1963-72 (2018).  42. K. Meier, T. Laino, and A. Curioni, “Solid-State Electrolytes: Revealing the Mechanisms of Li-Ion Conduction in Tetragonal and Cubic LLZO by First-Principles Calculations,” J Phys Chem C, 118 [13] 6668-79 (2014).  43. R. Jalem, Y. Yamamoto, H. Shiiba, M. Nakayama, H. Munakata, T. Kasuga, and K. Kanamura, “Concerted Migration Mechanism in the Li Ion Dynamics of Garnet-Type Li7La3Zr2O12
,” Chem Mater, 25 [3] 425-30 (2013).  44. C. Chen, Z. Lu, and F. Ciucci, “Data Mining of Molecular Dynamics Data Reveals Li Diffusion Characteristics in Garnet Li7La3Zr2O12
,” Sci Rep, 7 40769(2017).   45. Y. Zhang, F. Chen, and T. Rong, “Field Assisted Sintering of Dense Al-Substituted Cubic Phase Li7La3Zr2O12 Solid Electrolytes,” J Power Sources, 268 [3] 960-64 (2014).  46. M. Botros, R. Djenadic, and O. Clemens, “Field Assisted Sintering of Fine-Grained Li7-3xLa3Zr2AlxO12 Solid Electrolyte and the Influence of the Microstructure on the Electrochemical Performance,” J Power Sources, 309 108-15 (2016).  47. Y. Zhang, F. Chen, and R. Tu, “Effect of Lithium Ion Concentration on the Microstructure Evolution and its Association with the Ionic Conductivity of Cubic Garnet-Type Nominal Li7Al0.25La3Zr2O12 Solid Electrolytes,” Solid State Ionic, 284 53-60 (2015).  48. Y. Zhang, J. Cai, and F. Chen, “Preparation of Cubic Li7La3Zr2O12 Solid Electrolyte Using a Nano-Sized Core-Shell Structured Precursor,” J Alloys Compd, 644 793-98 (2015).  49. C. Shao, H. Liu, and Z. Yu, “Structure and Ionic Conductivity of Cubic Li7La3Zr2O12 Solid Electrolyte Prepared by Chemical Co-Precipitation Method,” Solid State Ionics, 287 13-6 (2016).  50. T. Yang, ZD. Gordon, and Y. Li, “Nanostructured Garnet-Type Solid Electrolytes for Lithium Batteries: Electro-spinning Synthesis of Li7La3Zr2O12 Nanowires and Particle Size-Dependent Phase Transformation,” J Phys Chem C, 119 [27] 14947-53 (2015).  51. R. Djenadic, M. Botros, and C. Benel, “Nebulized Spray Pyrolysis of Al-Doped Li7La3Zr2O12 Solid Electrolyte for Battery Applications,” Solid State Ionics, 263 [10] 49-56 (2014).  52. E. Rangasamy, J. Wolfenstine, and J. Sakamoto, “The Role of Al and Li Concentration on the Formation of Cubic Garnet Solid Electrolyte of Nominal Composition Li7La3Zr2O12
,” Solid State Ionics, 206 28-32 (2012).  53. K. Liu, JT. Ma, and CA. Wang, “Excess Lithium Salt Functions More than Compensating for Lithium Loss when Synthesizing Li6.5La3Ta0.5Zr1.5O12 in Alumina Crucible,” J Power Sources, 260 109-14 (2014).  54. JL. Allen, J. Wolfenstine, and E. Rangasamy, “Effect of Substitution (Ta, Al, Ga) on the Conductivity of Li7La3Zr2O12
,” J Power Sources, 206 [1] 315-19 (2012).  55. JM. Lee, T. Kim, and SW. Baek, “High Lithium Ion Conductivity of Li7La3Zr2O12 Synthesized by Solid State Reaction,” Solid State Ionics, 258 [5] 13-7 (2014).  56. Y. Li, JT. Han, and C. Wang, “Optimizing Li+ Conductivity in a Garnet Framework,” J Mater Chem, 22 [30] 15357-61 (2012).  57. J. Gai, and E. Zhao, “Improving the Li-Ion Conductivity and Air Stability of Cubic Li7La3Zr2O12 by the Co-Doping of Nb, Y,” J Eur Ceram Soc, 38 [4] 1673-78 (2018).  58. JF. Wu, EY. Chen, and Y. Yu, “Gallium-Doped Li7La3Zr2O12 Garnet-Type Electrolytes with High Lithium-Ion Conductivity,” ACS Appl Mater Interfaces, 9 [2] 1542-52 (2017).  59. YQ. Li, Z. Wang, and CL. Li, “Densification and Ionic-Conduction Improvement of Lithium Garnet Solid Electrolytes by Flowing Oxygen Sintering,” J Power Sources, 248 642-46 (2014).  60. S. Ohta, T. Kobayashi, and T. Asaoka, “High Lithium Ionic Conductivity in the Garnet-Type Oxide Li7-X La3(Zr2-XNbX)O12 (X=0-2),” J Power Sources, 196 [6] 3342-45 (2011).  61. R. Murugan, S. Ramakumar, and N. Janani, “High Conductive Yttrium Doped Li7La3Zr2O12 Cubic Lithium Garnet,” Electrochem Commun, 13 [12] 1373-75 (2011).  62. L. Dhivya, N. Janani, B. Palanivel, and R. Murugan, “Li+ Transport Properties of W Substituted Li7La3Zr2O12 Cubic Lithium Garnets,” AIP Adv, 3 [8] 82115-21 (2013).  63. S. Ramakumar, L. Satyanarayana, SV. Manorama, and R. Murugan, “Structure and Li+ Dynamics of Sb-Doped Li7La3Zr2O12 Fast Lithium Ion Conductors,” Phys Chem Chem Phys, 15 [27] 11327-38 (2013).  64. C. Deviannapoorani, L. Dhivya, and S. Ramakumar, “Lithium Ion Transport Properties of High Conductive Tellurium Substituted Li7La3Zr2O12 Cubic Lithium Garnets,” J Power Sources, 240 18-25 (2013).  65. C. Deviannapoorani, S. Ramakumar, and N. Janani, “Synthesis of Lithium Garnets from La2Zr2O7 Pyrochlore,” Solid State Ionics, 283 123-30 (2015).  66. D. Wang, G. Zhong, and WK. Pang, “Toward Understanding the Lithium Transport Mechanism in Garnettype Solid Electrolytes: Li+ Ion Exchanges and Their Mobility at Octahedral/Tetrahedral Sites,” Chem Mater, 27 [19] 6650-59 (2015).  67. D. Rettenwander, R. Wagner, and A. Reyer, “Interface Instability of Fe-Stabilized Li7La3Zr2O12 versus Li Metal,” J Phys Chem C, 122 [7] 3780-85 (2018).  68. AR. Yoo, SA. Yoon, and YS. Kim, “Comparative Study on the Synthesis of Al-Doped Li6.2La3Zr2O12 Powder as a Solid Electrolyte Using Sol-Gel Synthesis and Solid-State Processing,” J Nanosci Nanotechnol, 16 [11] 11662-68 (2016).  69. H. Xie, Y. Li, and JB. Goodenough, “Low-Temperature Synthesis of Li7La3Zr2O12 with Cubic Garnet-Type Structure,” Mater Res Bull, 47 [5] 1229-32 (2012).  70. I. Kokal, M. Somer, and PHL. Notten, “Sol-Gel Synthesis and Lithium Ion Conductivity of Li7La3Zr2O12 with Garnet-Related Type Structure,” Solid State Ionic, 185 [1] 42-46 (2011).  71. J. Sakamoto, E. Rangasamy, and H. Kim, “Synthesis of Nano-Scale Fast Ion Conducting Cubic Li7La3Zr2O12
,” Nanotechnology, 24 [42] 424005(2013).  72. Y. Shimonishi, A. Toda, and Z. Tao, “Synthesis of Garnet-Type Li7-xLa3Zr2O12-1/2x and its Stability in Aqueous Solutions,” Solid State Ionics, 183 [1] 48-53 (2011).  73. Y. Jin, and PJ. Mcginn, “Al-Doped Li7La3Zr2O12 Synthesized by a Polymerized Complex Method,” J Power Sources, 196 [20] 8683-87 (2011).  74. Y. Li, JT. Han, and CA. Wang, “Ionic Distribution and Conductivity in Lithium Garnet Li7La3Zr2O12
,” J Power Sources, 209 [4] 278-81 (2012).  75. AA. Raskovalov, EA. Il’Ina, and BD. Antonov, “Structure and Transport Properties of Li7La3Zr2-0.75xAlxO12 Superionic Solid Electrolytes,” J Power Sources, 238 48-52 (2013).  76. R. Takano, K. Tadanaga, and A. Hayashi, “Low Temperature Synthesis of Al-Doped Li7La3Zr2O12 Solid Electrolyte by a Sol-Gel Process,” Solid State Ionics, 255 [2] 104-7 (2014).  77. N. Rosenkiewitz, J. Schuhmacher, and M. Bockmeyer, “Nitrogen-Free Sol-Gel Synthesis of Al-Substituted Cubic Garnet Li7La3Zr2O12 (LLZO),” J Power Sources, 278 104-8 (2015).  78. CH. Lee, GJ. Park, and JH. Choi, “Low Temperature Synthesis of Garnet Type Solid Electrolyte by Modified Polymer Complex Process and its Characterization,” Mater Res Bull, 83 309-15 (2016).  79. T. Takeuchi, H. Kageyama, and K. Nakanishi, “All-Solid-State Lithium Secondary Battery with Li2S-C Composite Positive Electrode Prepared by Spark,” J Electrochem Soc, 157 [11] A1196(2010).  80. R. Djenadic, M. Botros, and C. Benel, “Nebulized Spray Pyrolysis of Al-Doped Li7La3Zr2O12 Solid Electrolyte for Battery Applications,” Solid State Ionics, 263 49-56 (2014).  81. XP. Wang, Y. Xia, J. Hu, YP. Xia, Z. Zhuang, LJ. Guo, H. Lu, T. Zhang, and QF. Fang, “Phase Transition and Conductivity Improvement of Tetragonal Fast Lithium Ionic Electrolyte Li7La3Zr2O12
,” Solid State Ionics, 253 [12] 137-42 (2013).  82. J. Wolfenstine, J. Ratchford, and E. Rangasamy, “Synthesis and High Li-Ion Conductivity of Ga-Stabilized Cubic Li7La3Zr2O12
,” Mater Chem Phys, 134 [2-3] 571-75 (2012).  83. Y. Zhang, F. Chen, and J. Li, “Regulation Mechanism of Bottleneck Size on Li+, Migration Activation Energy in Garnet-Type Li7La3Zr2O12
,” Electrochim Acta, 261 137-42 (2018).  84. E. Rangasamy, J. Wolfenstine, and J. Sakamoto, “The Role of Al and Li Concentration on the Formation of Cubic Garnet Solid Electrolyte of Nominal Composition Li7La3Zr2O12
,” Solid State Ionics, 206 [1] 28-32 (2012).  85. J. Wolfenstine, J. Sakamoto, and JL. Allen, “Electron Microscopy Characterization of Hot-Pressed Al Substituted Li7La3Zr2O12
,” J Mater Sci, 47 [10] 4428-31 (2012).  86. A. Düvel, and A. Kuhn, “Mechanosynthesis of Solid Electrolytes: Preparation, Characterization, and Li Ion Transport Properties of Garnet-Type Al-Doped Li7La3Zr2O12 Crystallizing with Cubic Symmetry,” J Phys Chem C, 116 [29] 15192-202 (2012).  87. N. Bernstein, MD. Johannes, and K. Hoang, “Origin of the Structural Phase Transition in Li7La3Zr2O12
,” Phys Rev Lett, 109 [20] 205702(2012).  88. Y. Jin, and PJ. Mcginn, “Al-doped Li7La3Zr2O12 Synthesized by a Polymerized Complex Method,” J Power Sources, 196 [20] 8683-87 (2011).  89. J. Wolfenstine, J. Ratchford, and E. Rangasamy, “Synthesis and High Li-Ion Conductivity of Ga-Stabilized Cubic Li7La3Zr2O12
,” Mater Chem Phys, 134 [2-3] 571-75 (2012).  90. JF. Wu, EY. Chen, and Y. Yu, “Gallium-Doped Li7La3Zr2O12 Garnet-Type Electrolytes with High Lithium-Ion Conductivity,” ACS Appl Mater Interface, 9 [2] 1542-52 (2017).  91. D. Rettenwander, CA. Geiger, and G. Amthauer, “Synthesis and Crystal Chemistry of the Fast Li-Ion Conductor Li7La3Zr2O12 Doped with Fe,” Inorg Chem, 52 [14] 8005-9 (2013).  92. D. Rettenwander, CA. Geiger, and M. Tribus, “The Solubility and Site Preference of Fe3+ in Li7-3xFexLa3Zr2O12 Garnets,” J Solid State Chem, 119 266-71 (2015).  93. D. Rettenwander, “Interface Instability of Fe-Stabilized Li7La3Zr2O12 versus Li Metal,” J Phys Chem C, 122 [7] 3780-85 (2018).  94. M. Huang, A. Dumon, and CW. Nan, “Effect of Si, In and Ge Doping on High Ionic Conductivity of Li7La3Zr2O12
,” Electrochem Commun, 21 62-4 (2012).  95. Y-T. Chen, A. Jena, WK. Pang, VK. Peterson, H-S. Sheu, H. Chang, and R-S. Liu, “Voltammetric Enhancement of Li-Ion Conduction in Al-Doped Li7-xLa3Zr2O12 Solid Electrolyte,” J Phys Chem C, 121 [29] 15565-73 (2017).  96. H. Elshinawi, GW. Paterson, and DA. Maclaren, “Low-Temperature Densification of Al-Doped Li7La3Zr2O12: A Reliable and Controllable Synthesis of Fast-Ion Conducting Garnets,” J Mater Chem A, 5 [1] 319-29 (2016).  97. EJ. Cheng, A. Sharafi, and J. Sakamoto, “Intergranular Li Metal Propagation through Polycrystalline Li6.25Al0.25La3Zr2O12 Ceramic Electrolyte,” Electrochim Acta, 223 85-91 (2017).  98. L. Zhang, X. Zhan, and YT. Cheng, “Charge Transport in Electronic-Ionic Composites,” J Phys Chem Lett, 8 [21] 5385-89 (2017).  99. S. Kobi, and A. Mukhopadhyay, “Structural (in)Stability and Spontaneous Cracking of Li-La-Zirconate Cubic Garnet upon Exposure to Ambient Atmosphere,” J Eur Ceram Soc, 38 [14] 4707-18 (2018).  100. R. Kun, F. Langer, and MD. Piane, “Structural and Computational Assessment of the Influence of Wet-Chemical Post-Processing of the Al-Substituted Cubic Li7La3Zr2O12
,” ACS Appl Mater Interface, 10 [43] 37188-97 (2018).  101. C. Im, D. Park, H. Kim, and J. Lee, “Al-Incorporation into Li7La3Zr2O12 Solid Electrolyte Keeping Stabilized Cubic Phase for All-Solid-State Li Batteries,” J Energy Chem, 27 [5] 1501-8 (2018).  102. XJ. Lu, and DY. Yang, “Preparation of Garnet-Type Li7-3xAlxLa3Zr2O12 at Lower Temperature by Using Powders of Mixed Pre-treatment Conditions,” J Inorg Organomet Polym Mater, 28 [5] 2023-27 (2018).   103. JF. Nonemacher, C. Hüter, and H. Zheng, “Microstructure and Properties Investigation of Garnet Structured Li7La3Zr2O12 as Electrolyte for All-Solid-State Batteries,” Solid State Ionics, 321 126-34 (2018).  104. JK. Padarti, TT. Jupalli, and C. Hirayama, “Low-Temperature Processing of Garnet-Type Ion Conductive Cubic Li7La3Zr2O12, Powders for High Performance All Solid-Type Li-Ion Batteries,” J Taiwan Inst Chem Eng, 90 85-91 (2018).  105. WJ. Xue, YP. Yang, and QL. Yang, “The Effect of Sintering Process on Lithium Ionic Conductivity of Li6.4Al0.2La3Zr2O12 Garnet Produced by Solid-State Synthesis,” RSC Adv, 8 [24] 13083-88 (2018).  106. XX. Pan, JX. Wang, XH. Chang, YD. Li, and WB. Guan, “A Novel Solid-Liquid Route for Synthesizing Cubic Garnet Al-Substituted Li7La3Zr2O12
,” Solid State Ionics, 317 1-6 (2018).  107. M. Wang, and J. Sakamoto, “Correlating the Interface Resistance and Surface Adhesion of the Li Metal-Solid Electrolyte Interface,” J Power Sources, 377 7-11 (2018).  108. PC. Zhao, GP. Cao, and ZQ. Jin, “Self-Consolidation Mechanism and its Application in the Preparation of Al-Doped Cubic Li7La3Zr2O12
,” Mater Design, 139 65-71 (2018).  109. LC. Bernuy, WJ. Manalastas, and LDAJ. Miguel, “ChemInform Abstract: Atmosphere Controlled Processing of Ga-Substituted Garnets for High Li-Ion Conductivity Ceramics,” Chem Mater, 26 [12] 3610-17 (2014).  110. J. Wolfenstine, J. Ratchford, and E. Rangasamy, “Synthesis and High Li-Ion Conductivity of Ga-Stabilized Cubic Li7La3Zr2O12
,” Mater Chem Phys, 134 [2-3] 571-75 (2012).  111. R. Wagner, and D. Rettenwander, “Crystal Structure of Garnet-Related Li-Ion Conductor Li7-3xGaxLa3Zr2O12: Fast Li-Ion Conduction Caused by a Different Cubic Modification?,” Chem Mater, 28 [6] 1861-71 (2016).  112. SH. Yang, MY. Kim, and DH. Kim, “Ionic Conductivity of Ga-Doped LLZO Prepared Using Couette-Taylor Reactor for All-Solid Lithium Batteries,” J Ind Eng Chem, 56 422-27 (2017).  113. CL. Li, YF. Liu, and J. He, “Ga-Substituted Li7La3Zr2O12: An Investigation Based on Grain Coarsening in Garnet Type Lithium Ion Conductors,” J Alloys Compd, 695 3744-52 (2017).  114. F. Aguesse, W. Manalastas, and L. Buannic, “Investigating the Dendritic Growth during Full Cell Cycling of Garnet Electrolyte in Direct Contact with Li Metal,” ACS Appl Mater Interface, 9 [4] 3808-16 (2017).  115. RH. Brugge, AKO. Hekselman, A. Cavallaro, and FM. Pesci, “Garnet Electrolytes for Solid State Batteries: Visualization of Moisture-Induced Chemical Degradation and Revealing its Impact on the Li-Ion Dynamics,” Chem Mater, 30 [11] 3704-13 (2018).  116. M. Rawlence, AN. Filippin, A. Waeckerlin, and TY. Lin, “Effect of Gallium Substitution on Lithium Ion Conductivity and Phase Evolution in Sputtered Li7-3xGaxLa3Zr2O12 Thin Films,” ACS Appl Mater Inter, 10 [16] 13720-28 (2018).  117. S. Qin, X. Zhu, and Y. Jiang, “Extremely Dense Microstructure and Enhanced Ionic Conductivity in Hot-Isostatic Pressing Treated Cubic Garnet-Type Solid Electrolyte of Ga2O3-doped Li7La3Zr2O12
,” Funct Mater Lett, 11 [2] 1850029(2018).  118. S. Qin, X. Zhu, and Y. Jiang, “Growth of Self-Textured Ga3+-Substituted Li7La3Zr2O12 Ceramics by Solid State Reaction and their Significant Enhancement in Ionic Conductivity,” Appl Phys Lett, 112 [11] 113901(2018).  119. D. Rettenwander, “Structural and Electrochemical Consequences of Al and Ga Co substitution in Li7La3Zr2O12 Solid Electrolytes,” Chem Mater, 28 [7] 2384-92 (2016).  120. CA. Geiger, E. Alekseev, B. Lazic, M. Fisch, T. Armbruster, R. Langner, M. Fechtelkord, N. Kim, T. Pettke, and W. Weppner, “Crystal Chemistry and Stability of “Li7La3Zr2O12,” Garnet: A Fast Lithium-Ion Conductor,” Cheminform, 42 [13] 1089-97 (2015).
121. JF. Wu, WK. Pang, VK. Peterson, L. Wei, and X. Guo, “Garnet-Type Fast Li-Ion Conductors with High Ionic Conductivities for All-Solid-State Batteries,” ACS Appl Mater Interface, 9 [14] 12461-68 (2017).  122. A. Dumon, M. Huang, Y. Shen, and CW. Nan, “High Li Ion Conductivity in Strontium Doped Li7La3Zr2O12 Garnet,” Solid State Ionics, 243 [28] 36-41 (2013).  123. S. Narayanan, F. Ramezanipour, and V. Thangadurai, “Enhancing Li Ion Conductivity of Garnet-Type Li5La3Nb2O12 by Y- and Li-Codoping: Synthesis, Structure, Chemical Stability, and Transport Properties,” J Phys Chem C, 116 [38] 20154-62 (2012).  124. G. Larraz, A. Orera, J. Sanz, I. Sobrados, V. Diez-Gómez, and ML. Sanjuán, “NMR Study of Li Distribution in Li7-xHxLa3Zr2O12 Garnets,” J Mater Chem A, 3 [10] 5683-91 (2015).  125. M. Haritha, MB. Suresh, and R. Johnson, “Synthesis and Evaluation of Thermal, Electrical, and Electrochemical Properties of Ba0.5Sr0.5Co0.04Zn0.16Fe0.8O3-δ, as a Novel Cathode Material for IT-SOFC Applications,” Ionics, 18 [9] 891-98 (2012).  126. X. Chen, T. Wang, W. Lu, T. Cao, M. Xue, B. Li, and C. Zhang, “Synthesis of Ta and Ca Doped Li7La3Zr2O12, Solid-State Electrolyte via Simple Solution Method and its Application in Suppressing Shuttle Effect of Li-S Battery,” J Alloys Compd, 744 386-94 (2018).  127. X. Chen, T. Cao, M. Xue, H. Lv, B. Li, and C. Zhang, “Improved Room Temperature Ionic Conductivity of Ta and Ca doped Li7La3Zr2O12 via a Modified Solution Method,” Solid State Ionics, 314 92-7 (2018).  128. E. Rangasamy, J. Wolfenstine, and J. Allen, “The Effect of 24c-Site (A) Cation Substitution on the Tetragonal-Cubic Phase Transition in Li7-xLa3-xAxZr2O12 Garnet-Based Ceramic Electrolyte,” J Power Sources, 230 261-66 (2013).  129. RA. Jonson, and PJ. McGinn, “Tape Casting and Sintering of Li7La3Zr1.75Nb0.25Al0.1O12 with Li3BO3 Additions,” Solid State Ionics, 323 49-55 (2018).  130. T. Yang, Y. Li, and W. Wu, “The Synergistic Effect of Dual Substitution of Al and Sb on Structure and Ionic Conductivity of Li7La3Zr2O12 Ceramic,” Ceram Int, 44 [2] 1538-44 (2018).  131. SW. Baek, JM. Lee, and TY. Kim, “Garnet Related Lithium Ion Conductor Processed by Spark Plasma Sintering for All Solid State Batteries,” J Power Sources, 249 197-2062014?.  132. J. Sun, N. Zhao, and Y. Li, “A Rechargeable Li-Air Fuel Cell Battery Based on Garnet Solid Electrolytes,” Sci Rep, 7 412172017.   133. N. Janani, S. Ramakumar, and S. Kannan, “Optimization of Lithium Content and Sintering Aid for Maximized Li+ Conductivity and Density in Ta-Doped Li7La3Zr2O12
,” J Am Ceram Soc, 98 [7] 2039-46 (2015).  134. H. Buschmann, S. Berendts, and B. Mogwitz, “Lithium Metal Electrode Kinetics and Ionic Conductivity of the Solid Lithium Ion Conductors ‘Li7La3Zr2O12’ and Li7-xLa3Zr2-xTaxO12 with Garnet-Type Structure,” J Power Sources, 206 236-44 (2012).  135. K. Hayamizu, Y. Matsuda, and M. Matsui, “Lithium Ion Diffusion Measurements on a Garnet-Type Solid Conductor Li6.6La3Zr1.6Ta0.4O12 by Using a Pulsed-Gradient Spin-Echo NMR Method,” Solid State Nucl Magn Reson, 70 21-7 (2015).  136. M. Huang, W. Xu, Y. Shen, Y-H. Lin, and C-W. Nan, “X-Ray Absorption Near-Edge Spectroscopy Study on Ge-Doped Li7La3Zr2O12: Enhanced Ionic Conductivity and Defect Chemistry,” Electrochim Acta, 115 [3] 581-86 (2014).  137. X. Liu, Y. Li, T. Yang, and et al, “High Lithium Ionic Conductivity in the Garnet-Type Oxide Li7-2xLa3Zr2-xMoxO12 (x= 0?0.3) Ceramics by Sol-Gel Method,” J Am Ceram Soc, 100 [4] 1527-33 (2017).  138. Y. Li, Z. Wang, and Y. Cao, “W-Doped Li7La3Zr2O12 Ceramic Electrolytes for Solid State Li-ion Batteries,” Electrochim Acta, 180 37-42 (2015).  139. S. Ramakumar, L. Satyanarayana, and SV. Manorama, “Structure and Li+ Dynamics of Sb-Doped Li7La3Zr2O12 Fast Lithium Ion Conductors,” Phys Chem Chem Phys, 15 [27] 11327-38 (2013).  140. Z. Cao, W. Ren, and J. Liu, “Microstructure and Ionic Conductivity of Sb-doped Li7La3Zr2O12 Ceramics,” J Inorg Mater, 29 [2] 220-24 (2014).  141. S. Ohta, T. Kobayashi, and T. Asaoka, “High Lithium Ionic Conductivity in the Garnet-Type Oxide Li7-X La3(Zr2-X, NbX)O12 (X=0-2),” J Power Sources, 196 [6] 3342-45 (2011).  142. C. Liu, K. Rui, and C. Shen, “Reversible Ion Exchange and Structural Stability of Garnet-Type Nb-Doped Li7La3Zr2O12 in Water for Applications in Lithium Batteries,” J Power Sources, 282 286-93 (2015).  143. R. Murugan, S. Ramakumar, and N. Janani, “High Conductive Yttrium Doped Li7La3Zr2O12 Cubic Lithium Garnet,” Electrochem Commun, 13 [12] 1373-75 (2011).  144. J. Gai, E. Zhao, and F. Ma, “Improving the Li-Ion Conductivity and Air Stability of Cubic Li7La3Zr2O12 by the Co-Doping of Nb, Y on the Zr Site,” J Eur Ceram Soc, 38 [4] 1673-78 (2018).  145. S. Hu, Y. Li, and R. Yang, “Structure and Ionic Conductivity of Li7La3Zr2-xGexO12 Garnet-like Solid Electrolyte for All Solid State Lithium Ion Batteries,” Ceram Int, 44 [6] 6614-18 (2018).  146. A. Yoon Sang, NR. Oh, and YA. Ri, “Preparation and Characterization of Ta-substituted Li7La3Zr2-xO12 Garnet Solid Electrolyte by Sol-Gel Processing,” J Korean Ceram Soc, 54 [4] 278-84 (2017).   147. S. Song, B. Chen, and Y. Ruan, “Gd-Doped Li7La3Zr2O12 Garnet-Type Solid Electrolytes for All-Solid-State Li-Ion Batteries,” Electrochim Acta, 270 501-8 (2018).  148. X. Chen, T. Cao, and M. Xue, “Improved Room Temperature Ionic Conductivity of Ta and Ca Doped Li7La3Zr2O12 via a Modified Solution Method,” Solid State Ionics, 314 92-7 (2018).  149. C. Samson, K. He, and Y. Liu, “Electrochemical Performance of All-Solid-State Lithium Batteries Using Inorganic Lithium Garnets Particulate Reinforced PEO/LiClO4 Electrolyte,” Electrochim Acta, 253 430-38 (2017).  150. Y. Ren, T. Liu, and Y. Shen, “Garnet-Type Oxide Electrolyte with Novel Porous-Dense Bilayer Configuration for Rechargeable All-Solid-State Lithium Batteries,” Ionics, 23 [9] 2521-27 (2017).   151. S. Bernhard, R. Daniel, and B. Stefan, “Solid Electrolytes: Extremely Fast Charge Carriers in Garnet-Type Li6La3Zr-TaO12 Single Crystals,” Ann Phys, 529 [12] 1700140(2017).  152. E. Hany, C. Edmund, and A. Corr Serena, “Enhancement of the Lithium Ion Conductivity of Ta-Doped Li7La3Zr2O12 by Incorporation of Calcium,” Dalton Trans, 46 [29] 9415-19 (2017).  153. Y. Tang, Z. Luo, and T. Liu, “Effects of B2O3 on Microstructure and Ionic Conductivity of Li6.5La3Zr1.5Nb0.5O12 Solid Electrolyte,” Ceram Int, 43 [15] 11879-84 (2017).  154. M. He, Z. Cui, and C. Chen, “Formation of Self-Limited, Stable and Conductive Interfaces between Garnet Electrolytes and Lithium Anodes for Reversible Lithium Cycling in Solid-State Batteries,” J Mater Chem A, 6 [24] 11463-70 (2018).  155. H. Kyle, SA. Junio, and T. Venkataraman, “Characterization of Lithium-Rich Garnet-Type Li6.5La2.5Ba0.5ZrTaO12 for Beyond Intercalation Chemistry-Based Lithium-Ion Batteries,” Solid State Ionics, 318 71-81 (2018).  156. X. Huang, T. Xiu, and E. Badding Michael, “Two-Step Sintering Strategy to Prepare Dense Li-Garnet Electrolyte Ceramics with High Li+ Conductivity,” Ceram Int, 44 [5] 5660-67 (2018). 
|
|





























