Oxidation Behaviors of SiCf/SiC Composites Tested at High Temperature in Air by an Ablation Method
Article information
Abstract
Using the thermal ablation method, the oxidation behavior of SiCf/SiC composites was investigated in air and in the temperature range of 1,300°C to 2,000°C. At the relatively low temperature of 1,300°C, passive oxidation, which formed amorphous phase, predominantly occurred in the thermal ablation test. When the oxidation temperature increased, SiO (g) and CO (g) were formed by active oxidation and the dense oxide layer changed to a porous one by vaporization of gas phases. In the higher temperature oxidation test, both active oxidation due to SiO2 decomposition on the surface of the oxide layer and active/passive oxidation transition due to interfacial reaction between oxide and base materials such as SiC fiber and matrix phase simultaneously occurred. This was another cause of high temperature degradation of SiCf/SiC composites.
1. Introduction
SiC ceramics exhibit outstanding high temperature stability and radiation resistance, so they have been considered for aerospace, gas turbine engines, cogeneration gas turbines, heat exchangers, and nuclear reactor core component materials.1–3) However, material property degradation occurs through the formation of oxides from passive and active oxidation, evaporation, reaction between oxides and base material, contaminants of the atmosphere, and corrosion due to the high temperature vapor under high temperature oxidation atmosphere.4,5) To overcome these causes of degradation, material selection and process/evaluation techniques are being developed to form an environment barrier coating (EBC).6,7) Generally, oxidation of SiC is explained through the formation of SiO2 layers (weight increase, reaction (1) and (2)) from the passive oxidation and volatilization of SiC (weight decrease, reactions (3) and (4)). Also, various studies are being carried out because of the effects of the transitions of the two reaction mechanisms on the SiC base material (matrix and fiber) property degradation and the oxide layer interface reaction (reactions (4) ~ (6)) on the SiC material property degradation.4,8–11)
Active oxidation at high temperatures was observed to be a complex phenomenon in which degradation occurs due to vaporization of the oxide layer and reactions of the base material and oxide layer. While volatilization due to formation of gaseous SiO and CO can be simply explained through reaction reaction (3), further understanding of the SiO(g) volatilization and SiO2(s) formation due to the interface reaction of the SiC and SiO2, as shown in reactions (4) ~ (6) for high temperatures, is necessary.4,9,10) According to the thermodynamic calculation results reported by Harder et al.11) on SiC(-Si, -C)/SiO2, considerable pressure occurs at the SiC/SiO2 interface, such that the SiO2 layer is damaged. Active oxidation of SiO2 occurs with the consumption of SiO2 due to the vaporization of the SiO2 layer, formation of gas pressure from the interface reaction, and interface reaction. When considering the pressure measured at 1500°C, the effect of the vaporization of SiO2+ is insignificant and the interface reaction is an important mechanism of active oxidation. Thus, an increase in the oxidation temperature leads to damage of the SiO2 layer due to consumption of SiO2 and gas pressure increase from the interface reaction of SiC and SiO2.
In this study, property changes of the composite and oxide layer produced through high temperature thermal ablation at 1300 ~ 2000°C for the SiCf/SiC composite with the interphase of PyC were analyzed. Ablation test was carried out under air, where the effects of the contaminants and high temperature steam were excluded and only the oxidation behavior due to the SiCf/SiC composite from air was considered. Since the active/passive oxidation transition due to the reaction between the formed oxide layer and the base material SiC simultaneously has an impact with the active/passive oxidation due to the surface reaction for the high temperature oxidation SiC, the oxidation behaviors of the composite matrix and fiber were investigated with a focus on both impacts. Moreover, the SiC plate fabricated using chemical vapor deposition (CVD) underwent thermal ablation with the same conditions, allowing us to compare with the composite oxidation behaviors as reference tests.
2. Experimental Procedure
In order to fabricate a plate composite for oxidation testing, SiC fabric with 2-dimensional weave using Tyranno SA3TM fiber (Ube, Ltd. Japan) was cut to dimensions of 200 × 200 mm and CVD was used to coat PyC on the the SiC fibers as the interphase, followed by an additional coating of SiC layer to prevent any reaction with the sintering aid that could occur during hot pressing for matrix densification. The PyC layer was deposited with a thickness of 200 nm under the conditions of 1,100°C and 20 ~ 50 torr with CH4. The SiC layer was deposited at 1,000°C using methyltrichlorosilane (MTS, CH3SiCl3) as the starting material. The matrix filling was performed using commercial β-SiC powder (4620 KE, Nano Amor Inc., USA; 97.5% purity, β phase content in SiC: 85%, fabrication method: plasma CVD) with 12 wt% Al2O3-Y2O3 sintering additive by AC-electrophoretic deposition (EPD). The details of the EPD were found in the literature.12) Twenty layers of SiC fiber with β-SiC embedded in the matrix using EPD were stacked to carry out the hot pressing, which was conducted under an argon atmosphere for 2 h at 1750°C and a pressure of 20 MPa. The sheets prepared by hot pressing were cut to dimensions of 30 × 30 mm for the test specimens. For the oxidation test, the ablation tests were carried out under a kerosene-oxygen flame. The temperature of the reference specimen was measured and the flow rate of the fuel and oxygen, as well as the distance between the torch tip and the specimens, were adjusted to meet the target temperature. In the experiment, fuel pressure of 0.12 MPa and flow rate of 0.08 L/min were used. The oxygen pressure and flow rate were 0.22 MPa and 300 L/min, respectively, while the angle between the flame and the specimen was 90°. Temperature measurement during the ablation test was performed using a pyrometer; the temperature of the center point where the flame and the specimen made contact was measured. For the secondary measurement, a thermocouple was put in contact with the center of the rear side of the specimen for reference. The ablation temperature range was 1300°C ~ 2000°C and the oxidation time was 30 min. Also, the SiC plates fabricated using CVD with the same dimensions underwent thermal ablation under the same conditions as the reference specimens. To investigate the oxidation behavior, weight change measurement, elemental analysis through microstructure observation using an optical microscope and scanning electron microscope (SEM, JEM-6300, JEOL, Japan), along with energy dispersive X-ray spectroscopy (EDS, Oxford INCA system, UK) and phase analysis using a high resolution powder X-ray diffractometer (SmartLab, Rigaku, Japan) and Raman analysis (FT-Raman Spectrometer, RFS 100/S, Bruker, Germany) were conducted.
3. Results and Discussion
3.1. Weight Change
Figure 1(a) shows the weight change of the oxidized SiCf/SiC composite according to the oxidation temperature. This result shows the specimen weight change, measured before and after the oxidation test. Although there is the possibility of error in the measurements, it was considered sufficient to analyze the oxidation behavior through the weight change trend data. The composite specimens experienced weight loss for oxidation temperatures up to 2000°C, and the amount of weight loss showed a tendency to decrease as the oxidation temperature increased. In contrast to the general trend in which SiC experiences an increase or decrease in weight due to active/passive oxidation under the high temperature oxidation atmosphere, only weight loss was observed because the oxidation evaporation amount of the PyC coating at the interface between the fiber and matrix was greater than the weight increase amount of the oxidation layer. The tendency of decreasing relative weight loss at higher oxidation temperatures was thought to be due to oxidation suppression by PyC from the self-sealing of SiO2 according to the oxidation of SiO. Further systematic analysis regarding this trend is necessary. This result shows that there was a limit in analyzing the oxidation behavior through evaluation of the weight change of the composite for the interface coated with PyC. Fig. 1(b) shows the results of an indirect method in which the weight change according to the oxidation temperature was measured after oxidation testing under the same conditions and using the same equipment as for the SiC plate fabricated using CVD. Unlike the composite, the weight change trend was one of weight increase and the weight increase amount decreased for temperatures above 1600°C. It was found that weight increase due to passive oxidation and weight loss due to active oxidation occurred simultaneously; the effect of active oxidation increased for temperatures above 1600°C.
3.2. Phase Analysis
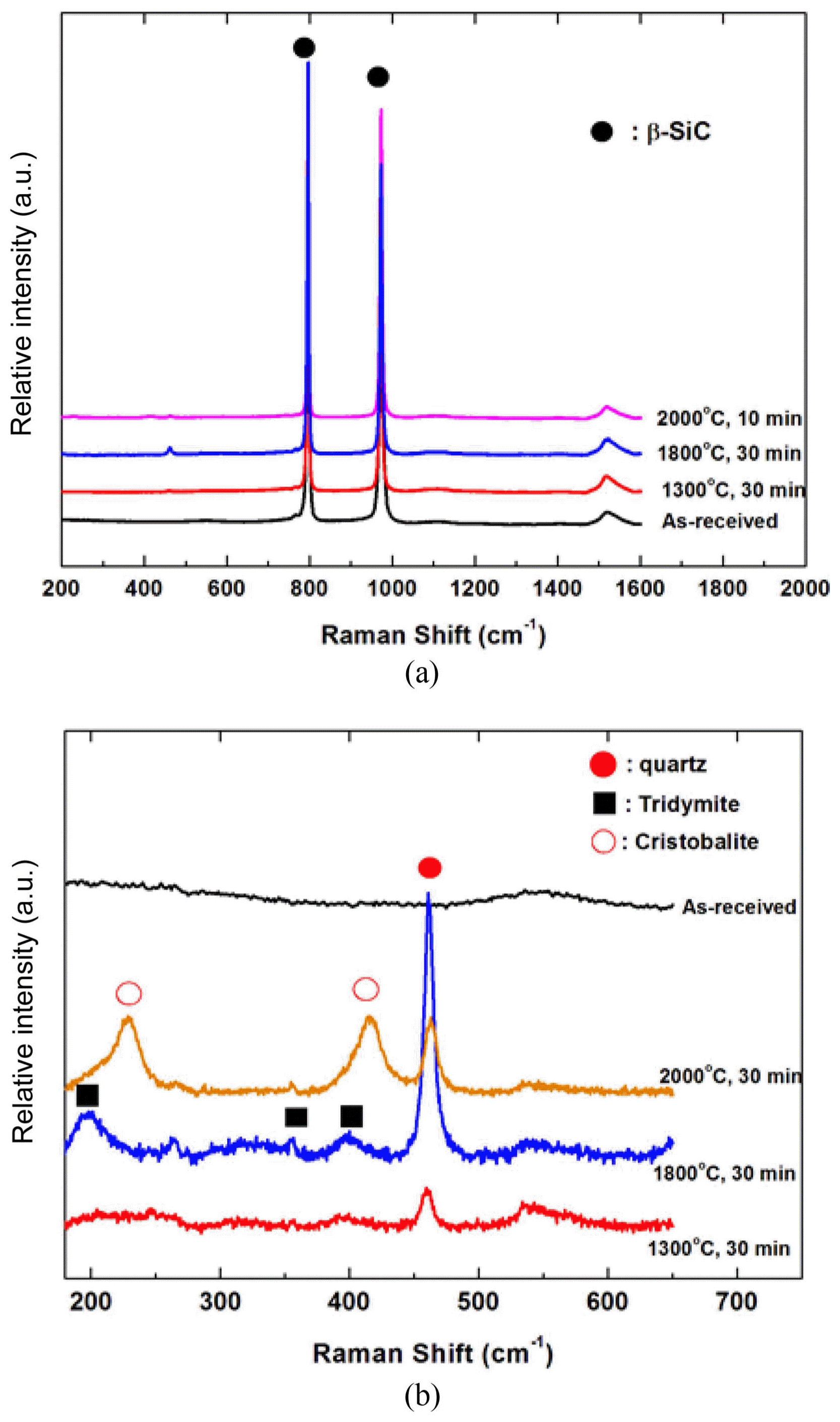
Figure 2 shows the phase analysis results from the X-ray diffraction analysis of the SiCf/SiC composite that underwent 30 min of thermal ablation oxidation testing at 1500 ~ 2000°C. The observed phases were β-SiC, a minute amount of α-SiC, and SiO2. α-SiC was similarly observed in all specimens as α-SiC was a component that existed in the starting ingredient, SiC powder; however, it was thought that this component does not have any significance in analyzing the oxidation behavior, so it was not further considered in the phase analysis of oxidized SiC. As can be observed in the analysis results, quartz, which is the low temperature phase of SiO2 was mainly observed for the oxidation layer SiO2; it was thought that this material was observed up to high temperatures as there was not sufficient time for a phase transition to the high temperature phase to occur as the oxidation time was 30 min. The high temperature phase cristobalite amount increased as the oxidation temperature increased. Raman spectra analysis was conducted for indepth analysis of the SiO2 oxidation layer; the results are shown in Fig. 3. Fig. 3(a) shows the results that were used to determine the presence of β-SiC in the peak shift 1600 cm−1 range; high intensity peaks at 796 cm−1 and 972 cm−1 were observed, showing the presence of β-SiC. The SiO2 peaks were relatively low compared to those of the SiC peaks, so it was difficult to analyze the SiO2 peaks when the peaks were shown together. Thus, Fig. 3(b) shows the results of a separate observation in the 200 ~ 600 cm−1 range, where SiO2 can be identified. As can be seen in the figure, no SiO2 peaks were observed before oxidation. According to the oxidation temperature, increases in the SiO2 polymorph peaks and oxide amount were observed for the oxidized specimen. The quartz peak was observed for the oxidized composite at 1300°C and broad peaks, considered to be amorphous phase, were also seen to exist. When the oxidation temperature increased, the intensity of the quartz peak increased, and the increase in the sharp peaks showed that the amount of crystalline SiO2 increased. The SiO2 phase was also observed as tridymite, which is the intermediate temperature phase of SiO2. For the specimen oxidized at 1,800°C, crystalline quartz and tridymite phases with sharp Raman peaks were observed, and high temperature phase cristobalite and quartz were observed as the major phases of SiO2 for the specimen oxidized at 2000°C. As discussed in the X-ray diffraction analysis results, the oxidation time was considered to be too short for SiO2 phase transition to occur, and the formation of the high temperature phase was observed according to the oxidation temperature.

X-ray diffraction peaks of SiCf/SiC composites oxidized in temperature range of 1,500 to 2,000°C in air for 30 min.
3.3. Microstructure Analysis
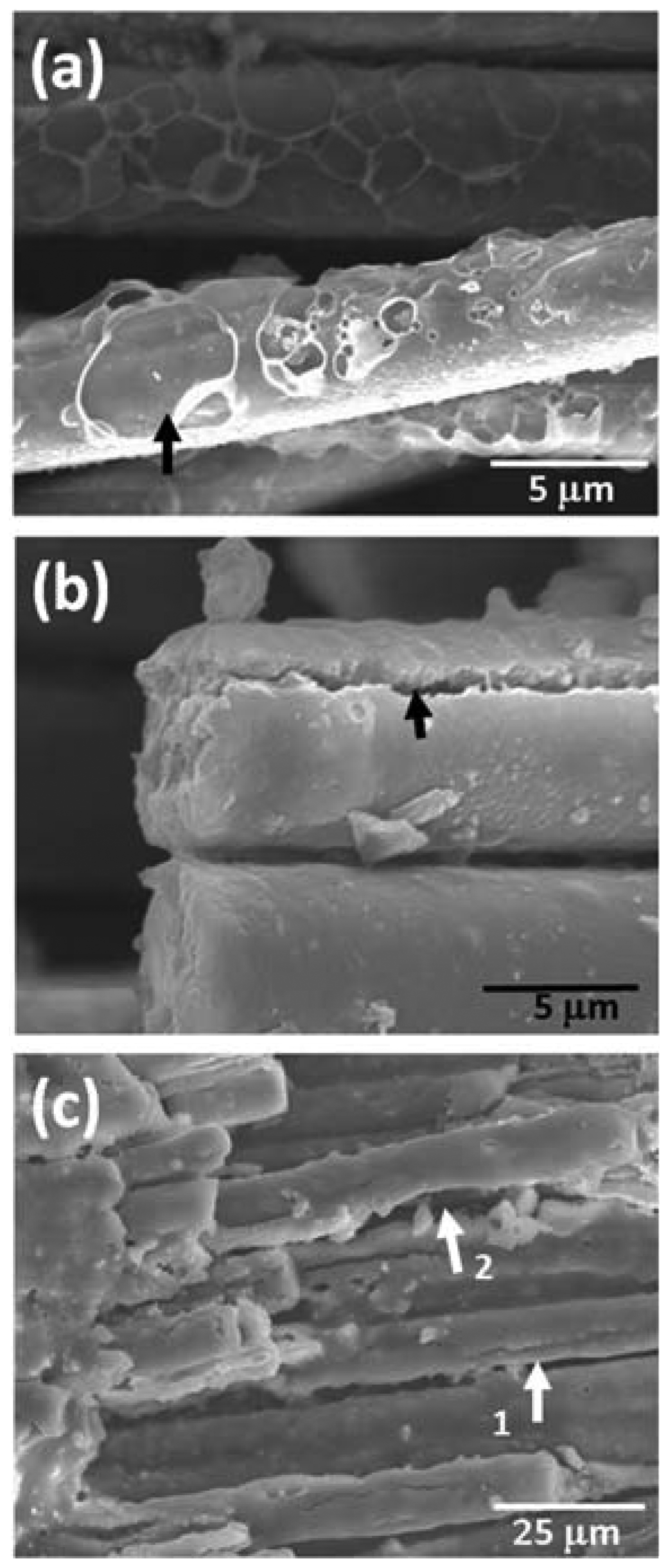
In order to analyze the properties of the oxidation layer, microstructure analysis through SEM observation and elemental analysis through EDS were conducted; the results are shown in Fig. 4. Fig. 4(a) shows the composite microstructure oxidized in air via 30 min of thermal ablation at 1500°C; Figs. 4(b), (c) and Figs. 4(d), (e) show the composite microstructures oxidized at 1,600°C and 1700°C, respectively. In addition, the compositions of the points shown in the microstructure were subjected to EDS element analysis and their results are shown together in the table. It was observed that a thick oxidation layer was formed in the matrix phase regardless of the oxidation temperature. However, as the oxidation temperature increased, numerous porous structures were observed in the oxidation layer surface. Point 1 in Fig. 4(a) shows a dense structure and elemental analysis of this point revealed that it constituted only of Si and O without detection of C. This result meant the formation of a dense oxidation layer and the progression of passive oxidation from the oxidation reaction of reactions (1) and (2). However, when the oxidation temperature increased, a relatively porous oxidation layer was observed as like points 3, 4 in Fig. 4(b) and point 7 in Fig. 4(d). In addition, C and O were simultaneously observed in the elemental analysis. These results were due to formation of the porous oxidation layers from volatilization of SiO(g) and CO(g) according to reaction (3) by the active oxidation. The simultaneous observation of C in the oxide layer seemed to be resulted from matrix phase located below a porous structure. Meanwhile, observation at the fiber level revealed the simultaneous presence of O and C because the oxidation layer was relatively thin compared to the matrix phase. This showed that the oxygen supply path for SiC fibers was not sufficiently available through the oxide layers on the matrix phase. However, as can be noted in Fig. 4(e), crack of the fiber oxidation surface was observed (marked with an arrow) and the amount of O also increased somewhat for the fiber oxidized at 1,700°C. This result was thought to be due to the simultaneous progression of the active and passive oxidation transitions by the interface reactions of SiC and SiO2 according to reactions (4) ~ (6) at high temperature and of the active oxidation according to reaction (3).4,8–11) Along with the active oxidation of the surface according to reaction (3), SiO(g) and CO(g) were produced due to the active oxidation at the interface between the fiber and the oxidation layer according to reactions (4) ~ (6). It is thought that these gas phases increased the internal pressure of the lower part of the oxide layer to cause cracking, and SiO(g) reacted with oxygen to generate additional SiO2, thereby increasing the O contents, relatively. In order to supplement the analysis of the reaction of the interface between the oxide layer and fiber, the high magnification microstructure of the composite fiber oxidized at 1700°C (Fig. 5(a), (b)) and the microstructure of the composite fiber oxidized at the higher temperature of 2000°C (Fig. 5(c)) were observed. In Fig. 5(a), traces of void formation (marked with an arrow) from SiO and CO gases by surface active oxidation of reaction (3) were observed. In Fig. 5(b), deep crack caused by the interface gas pressure rise from the SiO and CO gases produced from reactions of the SiC fiber and SiO2 oxidation layer according to reactions (4) ~ (6) were observed. On the other hand, the microstructure of the specimen oxidized at the higher temperature of 2,000°C, shown in Fig. 5(c), showed clearer degradation of the fiber. Not only was formation of cracks on the oxidation layer (marked with arrow 1) observed but reduction of the fiber diameter (marked with arrow 2) was also observed. This result suggested that SiC was consumed by active/passive oxidation transition between fiber and oxide layer as the oxidation temperature increased.

Microstructures with EDS analysis results of SiCf/SiC composites oxidized at 1,500°C (a), 1,600°C (b), (c), and 1,700°C (d), (e) in air for 30 min.
4. Conclusions
Thermal ablation oxidation test of SiCf/SiC composite at 1,300°C in air revealed that passive oxidation occurs, including amorphous formation, but when the oxidation temperature increased, the formation of crystalline oxidation layer and formation of SiO(g) and CO(g) due to decomposition of SiO2 on the oxidation layer surface by active oxidation became active and the density of the oxidation layer decreased.
In the higher temperature oxidation test, active oxidation from the decomposition of SiO2 on the oxidation layer surface and active/passive oxidation transition due to the interfacial reaction of the SiC base material and oxidation layer occurred simultaneously, becoming a different cause of SiC fiber degradation.
Microstructure observations revealed deep cracks in the SiC fiber oxide layer and reduction in the diameter of the SiC fibers, which were thought to be attributed to the reaction between the oxide layer and the SiC fibers at the oxidation temperatures above 1,700°C.
Acknowledgments
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean Government (MSIT)(No. 2017M2A8A401742). The authors would like to thank Professor Dang-Hyuk Yoon of Yeungnam University for helping with the specimen fabrication and Daeyang Industry for conducting the thermal ablation test.