Reductive Dissolution of Spinel-Type Iron Oxide by N2H4–Cu(I)–HNO3
Article information
Abstract
A N2H4–Cu(I)–HNO3 solution was used to dissolve magnetite powders and a simulated oxide film on Inconel 600. The addition of Cu(I) ions to N2H4–HNO3 increased the dissolution rate of magnetite, and the reaction rate was found to depend on the solution pH, temperature, and [N2H4]. The dissolution of magnetite in the N2H4–Cu(I)–HNO3 solution followed the contracting core law. This suggests that the complexes of [Cu+(N2H4)] formed in the solution increased the dissolution rate. The dissolution reaction is explained by the complex formation, adsorption of the complexes onto the surface ferric ions of magnetite, and the effective electron transfer from the complexes to ferric ions. The oxide film formed on Inconel 600 is satisfactorily dissolved through the successive iteration of oxidation and reductive dissolution steps.
1. Introduction
Magnetite (Fe3O4) has a spinel structure. Its unit cell contains 32 cation octahedral sites and 64 tetrahedral sites. One eighth of the tetrahedral sites and one fourth of the octahedral sites are occupied by trivalent Fe ions, and one fourth of the octahedral sites are occupied by divalent Fe ions.1) Ni, Cr-substituted magnetite is the main iron oxide that forms on the surface of iron-based metals, especially when in contact with the coolants used in pressurized water reactors (PWRs). The best way to remove radionuclides such as Co-60 in the oxide layer is to dissolve the oxide without affecting the base metal.
Many solution formulations have been developed to decontaminate the internal loops of nuclear power plants. A formulation consisting of nitrilotriacetic acid (NTA) and hydrazine at high temperature (160°C) was optimized and tested for its efficiency for dissolving oxides formed under boiling water reactor (BWR) conditions on SS-304 surfaces.2) This NTA-hydrazine formulation at 160°C could dissolve the oxides in a single step. Rodenas et al. measured the dissolution rates of NiO, CoO, ZnO, and Fe2O3 and their corresponding ferrites in oxalic acid at 70°C.3) The dissolution of simple oxides proceeded through the formation of surface metal oxalate complexes, followed by the transfer of surface complexes. They concluded that the most important factor determining the rates of dissolution of metal oxides was the lability of metal–oxygen bonds. Keny et al. evaluated the effects of radiation on the dissolution kinetics of magnetite and hematite in ethylenediaminetetraacetic acid (EDTA)-based and NTA-based dilute chemical-decontamination formulations. 4) EDTA-based formulations were more efficient for dissolving iron oxides than NTA-based formulations in unirradiated solutions. They also reported that the efficiency of dissolution was affected to a much greater extent in formulations containing EDTA and ascorbic acid compared with those containing NTA and gallic acid.
The dissolution of magnetite particles in solutions containing EDTA and Fe(II) was studied as a function of the total concentrations of EDTA and Fe(II).5) The results were interpreted in terms of fast solution and surface complexation processes, followed by slow heterogeneous electron transfer from adsorbed FeY2− to surface >Fe(III) centers and fast phase transfer of >Fe(II). The inhibitory effect of excess EDTA was also found to result from the competitive adsorption of FeY2− and EDTA. Segal and Sellers studied the reactions of solid iron oxides with aqueous reducing agents.6) The reaction of Fe2O3 with V(pic)3− was too rapid to be measured via sampling methods. They reported that one-electron reducing agents dissolved oxides containing >Fe(III), including insoluble nickel ferrite, and that the mechanisms of such reactions could be interpreted by analogy with homogeneous electron-transfer reactions. It is interesting to note that metal ions enhance dissolution in a way that is related to both its ability for complexation with ligands and the reducing power of surface >Fe(III) centers. Hydrazine not only acts as a reducing agent of metal ions but also forms a coordination compound with them. Furthermore, hydrazine is decomposed easily into nitrogen and water by hydrogen peroxide.
The objective of this study is to evaluate the dissolution capability of N2H4–Cu(I)–HNO3 on spinel-type oxides, such as magnetite, and the oxide film that forms on the surface of Inconel 600. The probable reactions involved in the dissolution of magnetite are also suggested.
2. Experimental Procedure
2.1. Magnetite dissolution
All the chemicals used in this study were of AR/GR grade, and all solutions were made in demineralized water. The synthesis of magnetite was initiated by precipitating ferrous ammonium sulfate at a pH of 11 using NaOH. The resulting hydroxide was calcined at 800°C in an inert atmosphere for 10 h. The prepared samples were thoroughly ground in an agate mortar and were characterized via X-ray diffraction (XRD) spectroscopy. Experiments on magnetite dissolution were carried out in a magnetically stirred Erlenmeyer flask on a hot plate. In all experiments, an amount of magnetite equivalent to 6.6 × 10−4 M (35.5 ppm) of iron was employed. Hydrazine monohydrate was used as the main dissolution agent; 65% HNO3 for pH control and Cu(NO3)2·3H2O were used as received. The solution was de-aerated for 30 min via bubbling with highly pure N2 gas. To prevent changes in mass transfer, all experiments were carried out at a constant stirring speed. The amounts of iron ions dissolved in the solutions were determined via atomic absorption analysis.
2.2. Identification of copper hydrazine complexes
The addition of 4.0 g of CuCl2 to a solution of 2.4 g of N2H4 in 11 ml of 1-M HCl produced an immediate light-green precipitate solution. Copper(I) hydrazine complexes were synthesized at 90°C for 10 min in a reduction atmosphere via a hydrothermal process. Because the synthesized compound oxidized easily in air, it was treated carefully. Analysis was performed using a far Fourier transform infrared (FT-IR) absorption spectrometer. The IR spectra of the complexes were recorded in the wavenumber range between 340 and 3400 cm−1.
2.3. Oxide film dissolution
Coupons (~ 7.6 cm2) of Inconel 600 polished up to 600 grit/P1200 using silicon carbide paper on an automatic grinder were used for the formation of the oxide film. A 4.5-L semiloop was filled with an aqueous solution containing LiOH (2.2 mg/L of Li), H3BO3 (650 mg/L of B), and N2H4 (90 mg/L). Coupons were placed in the semi-loop and the solution was circulated at 350°C for 30 days. The pressure in the semiloop was 2700 psi and the dissolved oxygen concentration was maintained at 10 ppb. XRD analysis of the oxide film scraped from the Inconel 600 coupons indicated that the produced film was essentially made of spinel-structured nickel chromium ferrite. The coupons were also treated with a solution containing HNO3 (0.32 g/L) and KMnO4 (1.0 g/L) at 95°C for 12 h before the reductive dissolution step. The amounts of dissolved iron ions in the reductive dissolution step and chromium ions in the oxidation step were analyzed. The surface morphology of the coupons was investigated via scanning electron microscopy (SEM) before and after the dissolution tests.
3. Results and Discussion
3.1. Magnetite dissolution
Figure 1 shows a plot of the dissolved fraction of magnetite vs. time under various [N2H4] at 95°C. The reproducibility of the dissolution data was within ± 2.8%. As shown in Fig. 1, the dissolved fraction of magnetite increased as [N2H4] in an acid solution increased. It took 6 h to dissolve the magnetite completely when [N2H4] was 0.07 M. This result is similar to that obtained for the dissolution kinetics of magnetite in EDTA- and NTA-based dilute chemical formulations. 4) Keny et al. reported that it took longer than 5 h to completely dissolve magnetite when the pH of the solution was 2.7–2.8 at 80°C. Baumgartner et al.7) proposed that the reductive dissolution of magnetite can be expressed as shown in Eq. (1).
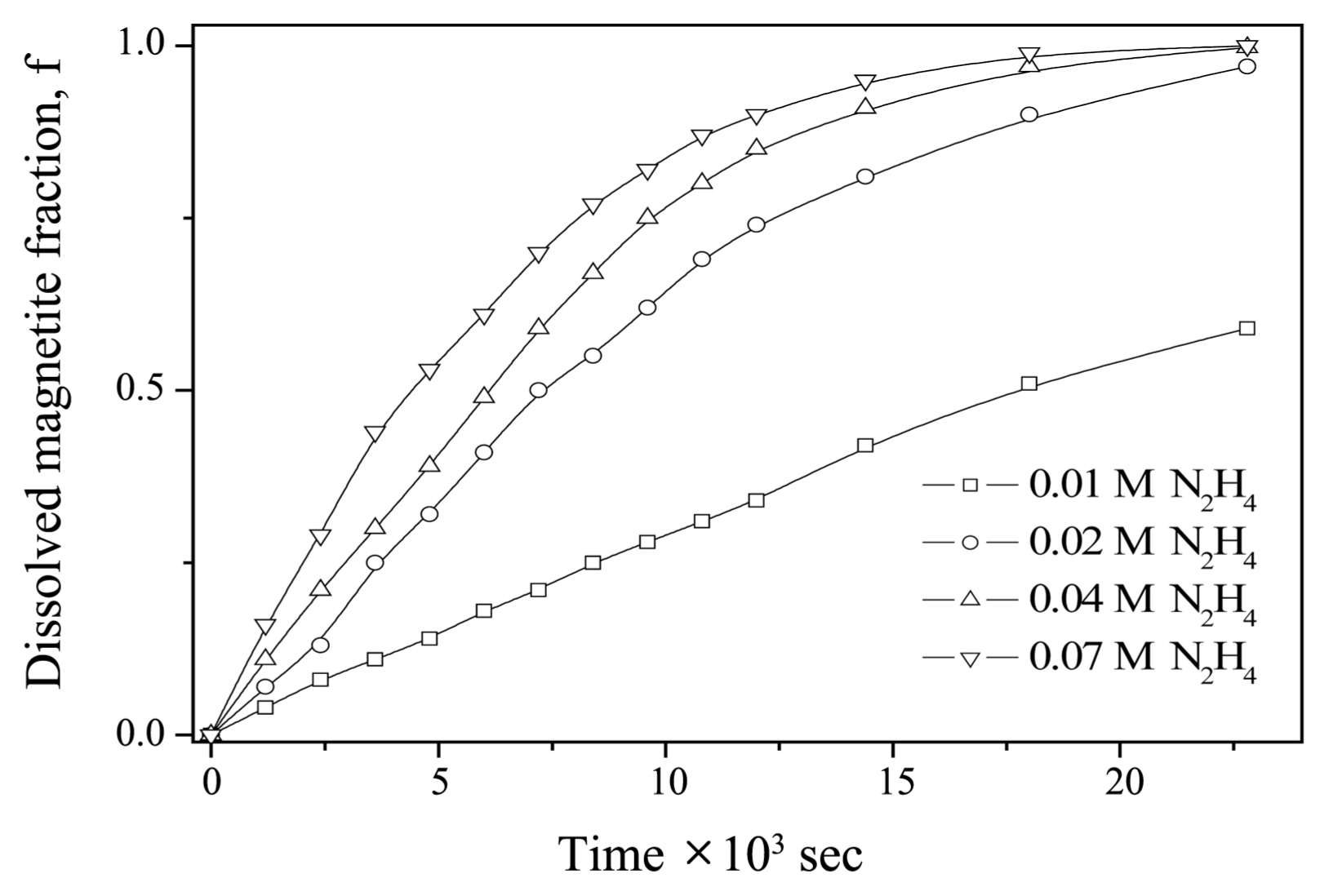
Dissolved fraction of magnetite (f) as a function of time at different [N2H4], pH = 3, T = 95°C and [Cu+] = 5 × 10−4 M.
Two explanations are possible in the N2H4–Cu(I)–H+ system. 1) Hydrazine itself is a reducing agent that can be used in an acid atmosphere to directly reduce the surface ferric ions of magnetite. 2) Hydrazine can also behave as a reducing agent of Cu(II) ions, and then Cu(I) ions efficiently reduce the surface ferric ions of magnetite.
A contracting core law of the form shown in Eq. (2) was found, where f is the fraction of dissolved iron in magnetite at time t and k is the apparent rate constant.
This law can be derived from the assumption that the dissolution rate is proportional to the instantaneous surface area and from an approximation of considering uniform and spherical particles. A plot of 1 − (1 − f)1/3 vs. time under various [N2H4] at 95°C is shown in Fig. 2. As shown in the figure, deviation from linearity occurs during the reaction period, except for the 0.01 M N2H4 system. The dissolution rate of magnetite increased with initial [Fe(II)] in EDTA, ethylenediaminedisuccinic acid (EDDS), and H2SO4 base solutions under the given conditions.5,8,9) After interpreting these results, Borghi et al. concluded that a faster rate could be achieved at an [EDTA] : [Fe(II)] ratio significantly larger than 1.5) The observed deviation from linearity may be explained by the increase of the dissolution rate caused by Fe(II) ions generated via the dissolution of magnetite. The presence of non-spherical magnetite particles cannot be excluded. Al-Mayouf et al. found that the dissolution rate of magnetite in EDDS solutions decreased with time.8) They suggested that the drop in the dissolution rate was due to the decrease in the surface area of dissolving particles. Fig. 2 shows linear correlations over the entire reaction time. This linearity is based on the control of surface chemical reactions for the dissolution of magnetite caused by the hydrazine base solution.
The effects of solution temperature on the dissolution of magnetite were investigated at [N2H4] = 0.02 M and a pH of 3. A plot of the dissolved fraction of magnetite vs. time under various temperatures is shown in Fig. 3. When the temperature was below 90°C, magnetite hardly dissolved. After a given time, the dissolved iron fraction was found to increase with solution temperature. After 4 h, more than 81% of the magnetite had dissolved at 95°C.

Dissolved fraction of magnetite (f) as a function of time at different temperatures, [N2H4] = 0.02 M pH = 3 and [Cu+] = 5 × 10−4 M.
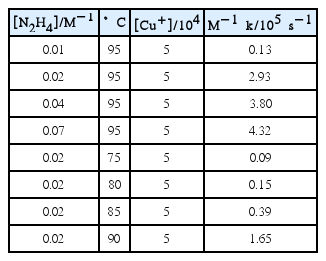
A plot of 1 − (1 − f )1/3 vs. time in the temperature range from 75°C to 95°C at 0.02 M [N2H4] is shown in Fig. 4. The dissolution rate was observed to be highly dependent on temperature because the k values steeply increased as temperature increased. The apparent rate constant k obtained for magnetite dissolution at various [N2H4] and temperature ranges are listed in Table 1. The obtained k values show that the dissolution rate depends on [N2H4] and temperature in the presence of 5 × 10−4 M [Cu(I)] at a pH of 3.

1 − (1−f )1/3 as a function of time in the temperature range from 75 to 95°C, [N2H4] = 0.02 M, pH = 3 and [Cu+] = 5 × 10−4 M.
The effect of [Cu(I)] and solution pH on the dissolution of magnetite was investigated. Fig. 5 shows the fraction of magnetite that had dissolved over the 6 h of reaction against [Cu(I)] at a constant [N2H4] and 95°C. At a pH of 2, the dissolved fraction of magnetite increased slowly as [Cu(I)] increased from 0 to 2.0 × 10−3 M. Above 2.0 × 10−3 M, the dissolved fraction of magnetite for any given time did not increase. At a pH of 3, the dissolved fraction of magnetite increased steeply as [Cu(I)] increased from 0 to 2.5 × 10−4 M. Above 2.5 × 10−4 M, the dissolved fraction of magnetite did not increase. Unlike the results for a pH of 2, under a pH of 3, magnetite was fully dissolved when [Cu(I)] exceeded 2.5 × 10−4 M. A total of 58% of the magnetite, however, dissolved over 6 h at a pH of 2.

Dissolved fraction of magnetite (f) over 6 h’ reaction as a function of [Cu+] at pH 2 and 3, [N2H4] = 0.07 M and T = 95°C.
The effect of [Cu(I)] on the dissolution of magnetite can be interpreted in terms of the following hydrolysis reaction:10)
where Cu2O(s) represents cuprous oxide. The concentration of soluble cuprous ions decreases as solution pH increases. Theoretically, the maximum [Cu(I)] at pH 3 is 1.45 × 10−4 M and at pH 2 is 1.45 × 10−3 M. A hydrolysis reaction from the red-colored cubic-structured oxide to yellow-colored Cu(OH) also occurs. Although a quantitative explanation is difficult, soluble Cu(I) ions greatly increase the dissolution rate of magnetite below a certain concentration for the given solution pH values.
The effects of pH on the dissolution of magnetite may be explained in terms of the half decomposition reaction of hydrazine [Eq. (4)]; its oxidation potential changes can be described by the familiar Nernst equation [Eq. (5)].
where E0 (1.17 V) is the standard oxidation potential of N2H4,11) F is the Faraday constant, R is the gas constant, and T is the absolute temperature. As expressed in Eq. (5), the oxidation potential increases with an increase in temperature, pH, and [N2H4]. The observed increase in the dissolution rate can be explained via the increase in the oxidation potential of hydrazine in this system. The acidic dissolution of magnetite is negligible, and Eq. (5) is qualitatively valid within the experimental range considered. When Cu(I) ions are excluded from N2H4–Cu(I)–HNO3, only 28% of magnetite dissolved over 6 h at 95°C at a pH of 3. In a separate test, it was also found that Cu(I) ions themselves barely dissolved magnetite in HNO3.
3.2. Identification of copper hydrazine bonding and its dissolution mechanism
Figure 6 shows a plot of charge vs. time under various incubation conditions. The charge values were obtained by integrating the electric current values measured via chronoamperometry. Fe3O4 particles deposited on the surface of indium-tin oxide (ITO) were used as the working electrode. After incubation under various solution conditions, all samples exhibited an increase in charge over time. The charge of the 0.3 mM Cu(NO3)2 + 30 mM N2H4 and 0.3 mM Cu(NO3)2 + 30 mM NH3BH3 systems increased significantly after incubation. This means that Cu2+ + N2H4 and Cu2+ + NH3BH3 systems can dissolve Fe3O4 efficiently.
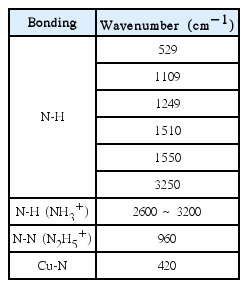
Figure 7 shows the FT-IR spectrum of a synthesized solid-state copper(I) hydrazine complex made from N2H4–Cu(I)–HCl. N–H stretching frequencies were observed at 529, 1109, 1249, 1510, 1550, and 3200 cm−1. The N–H stretching frequency of NH3+ was also observed in the range of 2600–3300 cm−1. The N–N stretching frequency observed at 960 cm−1 coincides with the N–N stretching frequency of hydrazinium ions (N2H5+) observed in the range of 958–965 cm−1. In particular, the Cu–N stretching frequency was also observed at 420 cm−1.12) The significant absorption peaks observed and their assignments are given in Table 2.
The reaction of hydrazine with Cu(II) chloride in acidic solutions has been shown to produce several complexes. It was reported that hydrazine behaves as a reducing agent, leading to white, diamagnetic cuprous complexes of (N2H4)CuCl and black paramagnetic mixed-valence tricopper (2I, II) complexes of (N2H5)2Cu3Cl6.13) Iskander et al. also reported that, at high temperatures, oxidation–reduction reactions occur between hydrazine and copper ions, yielding the formation of complexes with mixed oxidation states of copper.14) From our FT-IR spectral analysis, we predicted that complex formation occurs between Cu(I) ions and hydrazine in N2H4–Cu(I)–HNO3. This is related to the increase of the dissolution rate caused by the increase of [Cu(I)] under the given conditions. Hydrazine in acid solutions exists as hydrazinium ions. Because the dissolution rates of the systems under consideration are slow, Cu(II) ions are reduced to Cu(I) ions by the hydrazine in the acid solution during the dissolution of magnetite. In the meantime, Cu(I) ions form complexes with hydrazine. These complexes adsorbs on the surface Fe(III) ion sites of magnetite. Then, electron transfer from the complexes to Fe(III) ions occurs effectively. Fe(III) ions are reduced to Fe(II) ions and dissolve into the solution. To satisfy the electro-neutrality condition, the O2− ions of magnetite react with H+ ions and form water. The Cu(II) ion in the complex is reduced again to a Cu(I) ion by hydrazine. The above-described reaction scheme can be expressed as follows.
3.3. Oxide film dissolution
Figure 8 shows the amount of dissolved metal ions in the oxide film on the Inconel 600 surface vs. time during each step. When the oxidation pretreatment step was omitted, the reductive dissolution reaction was extremely slow. The reductive dissolution step was performed under a given concentration ([N2H4] = 0.04 M, [Cu(I)] = 5 × 10−4 M, and pH = 3) at 95°C. As shown in Fig. 8, iron ions continuously dissolved out from the oxide film in the first reductive dissolution step over 8 h. A total of 30.53 mg/cm2 of iron ions were dissolved in the first reductive dissolution step and 23.29 mg/cm2 of iron ions were dissolved in the second reductive dissolution step. The corrosion rate of Inconel 600, which was estimated using the weight loss method, was 1.5 × 10−5 mil/hr. It was previously reported that the difference in the dissolution behaviors of magnetite powder and the oxide film can, in such cases, be attributed to the participation of the underlying base metal, which significantly promotes the kinetics of dissolution.15) As the increase in the iron ion concentration became saturated after 8 h, the effect of the dissolution of the base metal on the dissolution rate of the oxide film was negligible in N2H4–Cu(I)–HNO3.
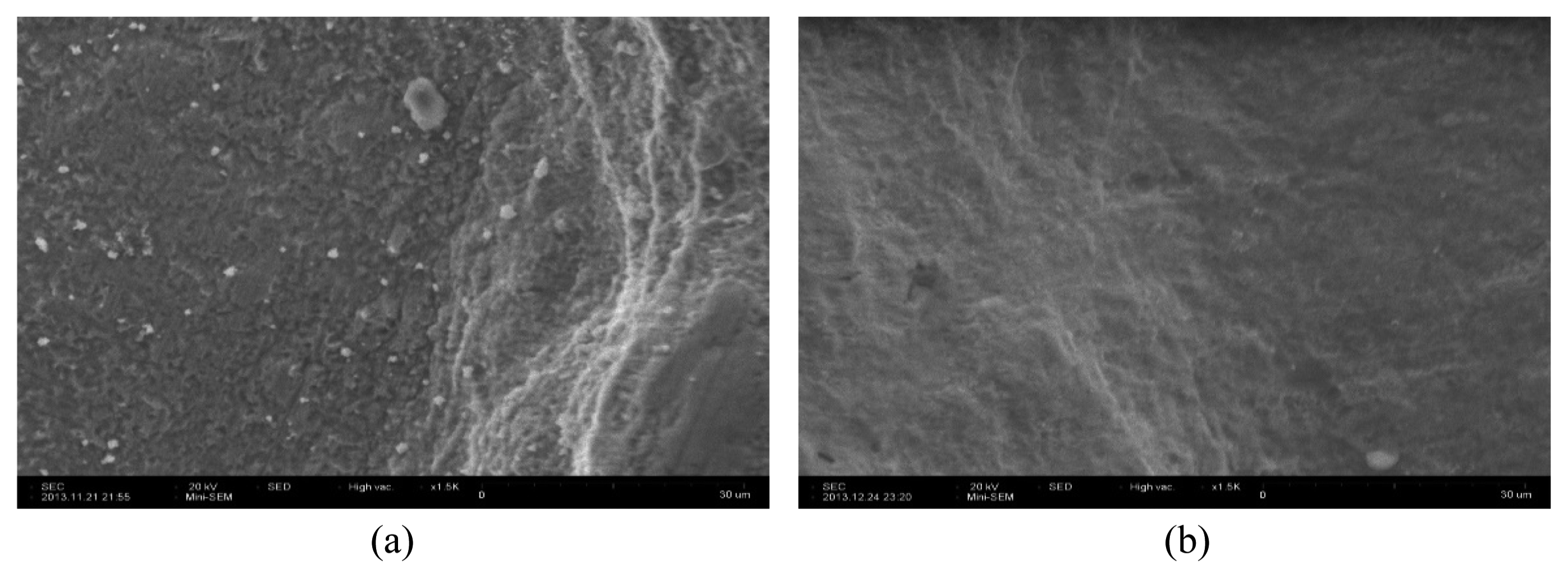
Figures 9 and 10 show images of the surface of the Inconel 600 specimen. As shown in Fig. 9, the color of the specimen before the dissolution test was black. After two cycles, the oxide layer was satisfactorily removed and the specimen exhibited a clean surface. As shown in Fig. 10, the adhered oxide particulates were fully removed and the surface of the Inconel 600 specimen was smooth.
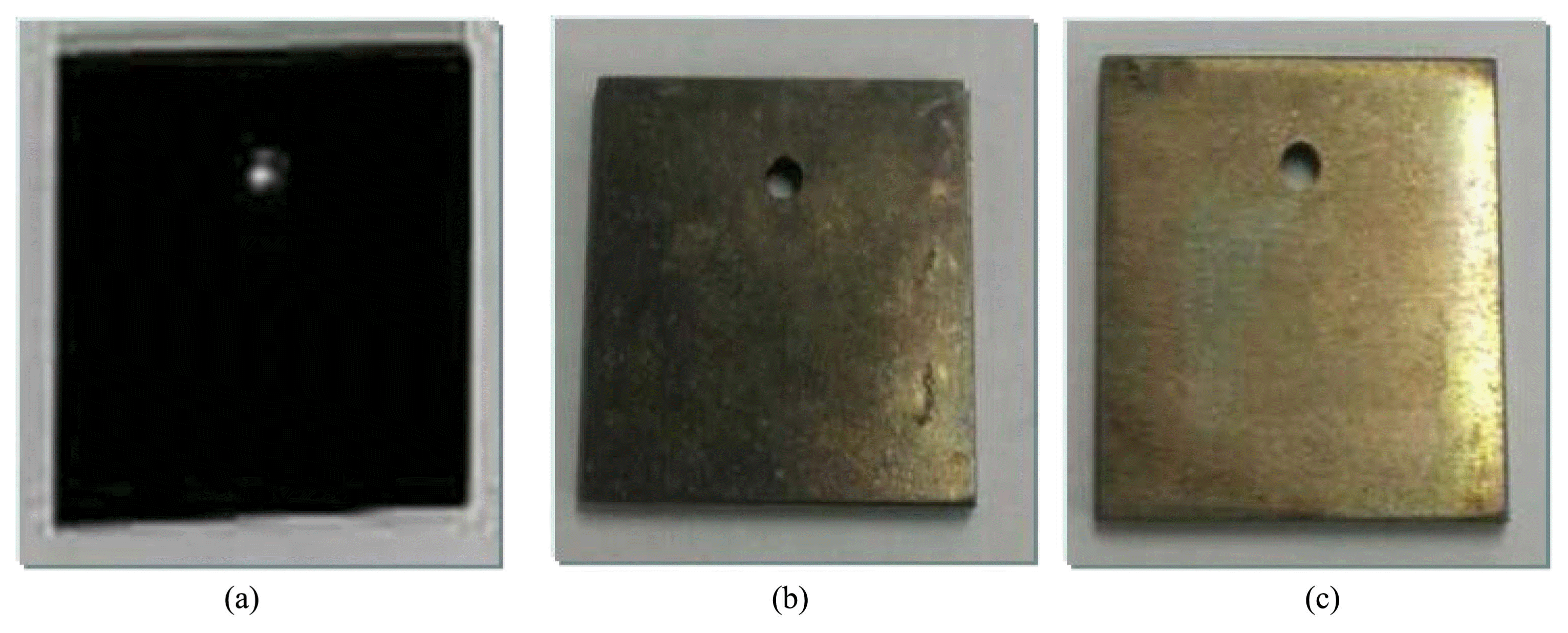
Photographs of Inconel 600 specimen: (a) before, and after (b) 2nd oxidation step and (c) 2nd reductive dissolution step.
4. Conclusions
The addition of copper ions to N2H4–HNO3 solutions offers a new pathway to dissolve magnetite; the main factors for dissolution can be explained by the oxidation potential changes of hydrazine and the catalytic property of copper ions. The formation of copper hydrazine complexes and its reaction pathway are suggested. The dissolution rate of magnetite particulates by N2H4–Cu(I)–HNO3 starts to slow down as reaction time increases. This was explained via the contracting core model. Our results indicate that the solution efficiently dissolves the simulated oxide film formed on the Inconel 600 surface. Because N2H4 reacts with hydrogen peroxide and easily decomposes into nitrogen gas and water, the generated volume of secondary waste can be significantly reduced by destroying the decontamination agent.16) It has already been reported that type-304 stainless-steel specimens radioactively contaminated under PWR water chemistry conditions were successfully decontaminated by N2H4–Cu+–HNO3.17) The prepared solution also exhibited good corrosion compatibility with Inconel 600. The newly formulated dilute chemical decontamination agent, which does not use conventional organic chelates such as EDTA, NTA, citric acid, or oxalic acid, will be a good option to decontaminate the internals of nuclear power plants.
Acknowledgments
The authors would like to express their appreciation to the National Research Foundation (NRF) of Korea for the award of a grant funded by the Ministry of Science and ICT (MIST) of the Republic of Korea, in support of the authors’ work through the Nuclear Research and Development Program (NRF-2017M2A8A5015144).