P2O5-ZnO-SiO2-R2O Glass Frit Materials for Hermetic Sealing of Dye-Sensitized Solar Cells
Article information
Abstract
P2O5-ZnO-SiO2-R2O glasses were synthesized as a sealing material for large scale dye-sensitized solar cells (DSSC). Compositional effects of P2O5 and ZnO were examined by varying their contents. Their viscosity and glass stability at sintering temperatures of less than 550°C were examined by flow button test. Glass transition temperature and structural change upon compositional change were investigated. Chemical stability against electrolyte was also examined by immersing the glasses in the electrolyte for 72 h at 85°C.
1. Introduction
Dye-sensitized solar cells (DSSC) have been extensively studied because of their advantages over conventional semiconductor-based solar cells, including simple manufacturing process, excellent colors, and low production cost.1,2) In particular, due to their various colors, DSSCs can be applied to the production of various products, and are expected to be applied for building integrated photo-voltaic cells (BIPV) because of their excellent transparency in comparison with that of other solar cells.3,4) Generally, a DSSC consists of a TiO2 electrode with a Ru-based dye deposited on it, a liquid electrolyte, and a fluorine doped tin-oxide (FTO). Surlyn, which is an organic material, is employed as a sealing material to separate individual cells, support the top and bottom glass substrates, and prevent gas and moisture infiltration outside of the DSSC.5) However, Surlyn has low thermal durability as an organic material; it also causes a decrease of the DSSC efficiency due to the infiltration of moisture and oxygen,6,7) preventing its application to large outdoor and indoor BIPV.
On the other hand, glass frit, a completely inorganic material having excellent thermal, chemical, and mechanical durability as well as excellent mechanical properties, has been extensively used as a sealing material for various displays including cathode-ray tubes (CRT), plasma display panels (PDP), and active matrix organic light emitting diodes (AMOLED). Therefore, studies have recently been conducted to replace the conventional Surlyn material with glass frit to secure high sealing performance.8,9,10) The application of glass frit to DSSC requires a glass material that does not react with an electrolyte, that may be sintered at a temperature lower than 550°C so as not to affect the DSSC substrate and the manufacturing process. The glass frit should also have high bond strength to the glass substrate and proper thermal expansion coefficient similar to that of the substrate. Lead silicate glasses can be easily sintered at low temperature and has been employed in conventional PDPs. However, lead silicate glass may damage the Pt-coated counter electrode inside a DSSC,11) due to Pb ions. Bi2O3-based glass has a sintering temperature as low as that of lead silicate glass. However, evaluation of the reaction between Bi2O3-based glass materials and DSSC electrolytes by Lee et al.8) showed that a reaction occurs with I−/I3−inside the electrolyte, and the reducing of Bi makes Bi2O3-based glass unsuitable for use as a separating and sealing material for DSSC. As a method of preparing low-temperature sealing glass materials, Cho et al.9) suggested laser sealing of a glass material produced by mixing TeO2 with V2O5-BaO-ZnO-B2O3-based glass. However, the method requires high production cost to be applied in the fabrication of large-area window-type BIPV devices, and crystallization may occur more easily. Recently, due to its excellent glass stability, V2O5-P2O5-ZnO-Sb2O3-based glass was developed as a material that can enable sealing by conventional thermal treatment method;10) however, V2O5-based glass lacks aesthetic features when applied to large-area devices because of the characteristics black color of V2O5-based glass materials.
In the present study, P2O5-ZnO-SiO2-R2O-based phosphate glass was employed as an amorphous inorganic sealing material because it enables the application of the conventional thermal process, which is more appropriate for the commercial production of large-area DSSC, and it can exhibit white color at the same time. The thermal and mechanical properties, depending on the P2O5 and ZnO contents, were investigated to determine an optimal composition for the hermetic sealing of DSSC. The glass forming ability, glass transition temperature, flow button size, and electrolyte durability depending on the composition were evaluated; the reactivity with electrolytes and the structural changes were tested and analyzed through X-ray diffraction (XRD), scanning electron microscope (SEM), Raman spectroscopy, and Fourier transform-infrared spectroscopy (FT-IR).
2. Experimental Procedure
The glass used in the present study had the composition of (70-x)P2O5-xZnO-30(SiO2+R2O)(x = 20, 22.5, 25, 27.5, 30) (R = Na, K). In addition, to improve the chemical durability of the glass, B2O3 and Al2O3 were added at maximum concentrations of 5 mol%. High-purity raw materials having purity of over 99.9% were weighed and mixed through ballmilling. Then, the resulting mixture was put into an alumina crucible to prepare the glass material. The glass was melted in an electric furnace at 1300°C for 1 h in air, and the melt was poured into a brass mold for quenching. The produced glass was pulverized using an agate mortar and screened to obtain powder having a diameter of less than 50 μm. To examine the glass stability and flowability, 4 g of the prepared glass frit was weighed and packed with an uniaxial press in a 12-Φ mold, followed by sintering at 550°C for 30 min. The reactivity with an electrolyte was tested by immersing the sintered samples into a container with a commercial DSSC electrolyte solution (NPN-15) in a thermostat water tank at 85°C for 72 h.
The glass transition temperature was measured by a differential scanning calorimetry (DSC, DSC60, Shimadzu, Japan) with the scan rate of 10°C/min. The crystalline phase of the sintered glass was examined via XRD (Mini flex 600, Rigaku, Japan). The thermal expansion coefficient was measured using a thermo-mechanical analyzer (TMA, TMA-60H, Shimadzu, Japan) in a range from room temperature to 200°C. To analyze the interfacial reaction with the electrolyte, the cross-section of the glass sample was examined by FE-SEM (TESCAN, MIRA LMH, Czech), and the composition was analyzed through energy dispersive X-ray spectroscopy (EDS). The structure of the prepared glass was investigated by Raman spectroscopy (ARAMIS, Horiba Jobin Yvon, France) and FT-IR spectroscopy (Nicolet iS50, Thermo Fisher Scientific, USA).
3. Results and Discussion
In the (70-x)P2O5-xZnO-30(SiO2+R2O)-based composition, the glass was prepared by varying x by 2.5 each time. To examine the flowability of the glass, a flow button test was performed with 4 g of the glass frit at 550°C. As shown in Fig. 1, transparent glass was successfully obtained when melted. After the 550°C flow button test, relatively good flowability and adhesion to the glass substrate were found by visual observation, with no crystallization on the glass surface. These results imply that the glass composition used in the present study has good properties as a sealing material for DSSC. Crystallization of the glass by thermal treatment was also examined by XRD. As the ZnO content increased, the flowability increased with the increase of the flow button size. After x was increased over 30, the flowability gradually decreased. The glass flowability increased as P2O5, a glass network former, was replaced by ZnO, reaching maximum flowability around x = 25.

Flow button test results of (70-x)P2O5-xZnO-30(SiO2+R2O) glasses with varying ZnO content (x) after sintering at 550°C for 30 min.
Figure 2 shows the glass transition temperature and the thermal expansion coefficient depending on the ZnO content. The glass transition temperatures of all the glass samples were lower than 460°C, confirming that the material is appropriate as a glass frit material for low sintering temperature process. The thermal expansion coefficient was in a range of 10.5 to 11.2 × 10−6/°C, which was similar to that of the FTO substrate used as a DSSC substrate (9 × 10−6/°C). Therefore, there may be only slight damage caused by the difference of the thermal expansion coefficient during the sintering process. In addition, as the ZnO content increased, the glass transition temperature and the thermal expansion coefficient decreased. This result may have been caused by the change of the glass structure depending on the content of P2O5 and ZnO, which will be discussed later. The general decrease of viscosity with the decrease of glass transition temperature is mostly due to the increase of structural flexibility of the glass, indicating that the concentration variation of P2O5 and ZnO affect the structural flexibility.
To examine the chemical durability of the obtained glass to the electrolyte, the sintered glass was immersed into the electrolyte solution at 85°C for 72 h as an accelerated condition; then, the surface condition and the cross-section were observed. As can be seen in the XRD pattern in Fig. 3, the surfaces of all the glass samples were not significantly changed by the electrolyte solution, showing good chemical stability. In addition, as Fig. 3 exhibits, the glass after the reaction showed a typical glass phase with almost no crystallization or precipitation resulting from possible chemical reaction with the electrolyte. Analysis of the XRD pattern showed that, although marginal dissolution of the glass component can be caused by the reaction with the electrolyte, but no apparent crystallization or precipitation has been appeared. This indicates that the glass material of the present study has relatively good chemical durability as a DSSC sealing material.

XRD results after reactivity test with electrolyte. Inset figure shows surface images before and after the test.
In order to inspect the reaction of the samples with the electrolyte in detail, the sample surface and cross-section were observed using SEM (Fig. 4). The glass surface was slightly damaged by the reaction with the electrolyte at x = 20 (ZnO content). However, as x increased, the surface damage gradually reduced, showing the least damage at x = 25. In addition, to verify the change of the composition by the chemical reaction depending on the depth from the cross-section, the compositions of the sample surface (①) and the inside of the sample (②) were investigated via EDS. The results showed that change was least at x = 25. Change of the composition may have occurred by the destruction of P2O5 network on the surface by the I−/I3− ions within the electrolyte, followed by the diffusion of P and Zn from the sample to the electrolyte. As Table 1 shows, the content of P was relatively higher on the surface than the inside while Zn shows lower content on the surface. It indicates relatively easy dissolution of Zn from the surface to the electrolyte. On the other hand, at x = 30, the Zn content was relatively high on the surface, probably because the dissolution rate of P and Zn has been changed with the increase of Zn content. Further study is required to understand the dissolution mechanism more clearly.

Surface and cross-sectional SEM image of (70-x)P2O5-xZnO-30(SiO2+R2O) glasses with varying ZnO content.
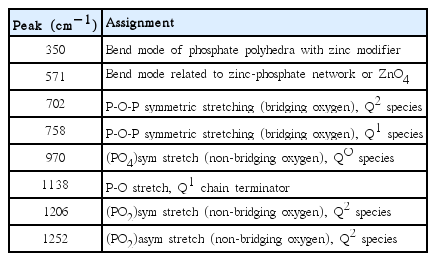
The change of the glass structure depending on the P2O5 and ZnO contents has been investigated with Raman spectroscopy and FT-IR spectroscopy. As shown in Fig. 5, characteristic Raman spectra of zincphosphate glass were found in all the glass samples. Table 2 shows the major Raman peaks obtained from the spectra. As the ZnO content (x) increased and the P2O5 content decreased, the peaks at 702 cm−1 and 1206 cm−1 shifted to 758 cm−1 and 1138 cm−1, respectively. This is due to the decrease of Q2 and increase of Q1 units within P-O-P chain in the glass structure,12–15) wherein Qn represents a unit having n-bridging oxygen (BO) atoms among [PO4] tetrahedral units.16) In other words, as the ZnO content increased, the glass network was modified by the breakage of P-O-P bonding, resulting in decreases of the glass transition temperature and the viscosity. The FT-IR results were similar to those of Raman spectroscopy. As shown in Fig. 6, the value of Q2 at 1266 cm−1 decreased, but the values of Q1 at 1042 cm−1 and 929 cm−1 relatively increased.
On the other hand, in contrast to the expectation that the glass transition temperature and the viscosity would decrease and thus that the chemical durability of the glass would drop due to the breakup of the glass network, the chemical stability was improved with the increase of the ZnO content, as shown in Fig. 6 and Table 1. It has generally been known that the addition of ZnO to a phosphate glass increases the value of Q1 and the number of non-bridging oxygens (NBOs) through the reaction (Q2+ZnO → 2Q1).12) As the structure is loosened by the increase of NBO in the glass, the glass transition temperature and the viscosity decrease, and the negative charges formed around Zn2+ and the alkali metal ions also increase in the glass structure. Brow16) reported that at an alkali oxide content of over 20 mol%, the formation of polyhedra composed of alkali ion and NBOs is preferred because the number of double bond oxygen atoms (terminal oxygen) per modifier ion is small in a phosphate tetrahedron; they also reported that the bonding of alkali ions with an adjacent Q3 tetrahedron is strengthened and the alkali polyhedra structure connects Q2 and Q3 in the glass structure. Therefore, the negative charges forming the alkali-polyhedra increase the resistance to the negative ions of I−/I3− and decrease the thermal expansion coefficient due to the increased binding force. However, further study is also required to understand the mechanism in detail.
Figure 7 shows the application of the glass frit obtained in the present study to an actual DSSC. The obtained glass frit was used to prepare a 300 mm × 300 mm DSSC; the cross-section was observed through SEM. As Fig. 7 shows, both glasses were well sealed, without any crystal phase or a secondary phase produced by sintering at the interface. Fig. 7 shows some cracks on the sealing glass after the sintering. The cracks, generated during the sintering process, may be reduced by optimizing the content of the organic vehicle added for the glass frit coating and the sintering temperature conditions. However, the bond of the sintered glass to the FTO layer was good, which confirmed the potential of the glass frit as a material for DSSC. The bond strength measured on a small-sized panel (15 × 15 mm) was as high as approximately 10,000 kg/cm2. Further studies are currently being carried out to evaluate the long-term durability of this material on a large-area cell of about 40 inches diagonal.
4. Conclusions
To secure the long term stability required by large-area DSSC applications, a (70-x)P2O5-xZnO-SiO2-R2O-based glass was prepared as an inorganic material-based amorphous sealing material that may replace Surlyn. As the ZnO content increased, the flowability increased and the glass transition temperature decreased. An accelerated test performed at 85°C for 72 h to verify the reactivity with an electrolyte showed that electrolyte reaction was delayed with the increase of the ZnO content. The glass transition temperature decreased with the increase of the ZnO content due to the breakage of P2O5 network along with the increase of Q1 structural units. However, the chemical resistance to the I−/I3− electrolyte was improved and thermal expansion coefficient is decreased, which may be due to the increase of NBOs and the formation of alkali polyhedra with the ZnO. These results show that the glass frit composition obtained in this study has relatively high stability against the DSSC electrolyte, excellent flowability and low-temperature sintering of 550°C, demonstrating its high potential for large scale DSSC applications.
Acknowledgements
The present study was supported by the Purchase-Conditioned New Product Development Project of the Small and Medium Business Administration (Grant No: 201410700001).