Photocatalytic Performance of CoS2-Graphene-TiO2 Ternary Composites for Reactive Black B (RBB) Degradation
Article information
Abstract
In this study we examined the photo-degradation efficiency of CoS2-G-TiO2 nanocomposites under visible light irritation using Reactive Black B (RBB) as standard dye, CoS2-G-TiO2 nanocomposites synthesized by facial microwave assist technique, and characterized by X-ray diffraction (XRD), scanning electron microscopy (SEM), transmission electron microscopy (TEM), and Raman spectroscopic analysis. Our results show the efficiency of the CoS2-G-TiO2 ternary nanocomposite is better than CoS2-G and TiO2-G nanocomposite. The degradation efficiency of CoS2-G-TiO2 nanocomposite was found approximately 89% of Reactive Black B (RBB) degraded after 180 min. Our results will open new way for the development of a new ternary nanocomposite photocatalytic application.
1. Introduction
According to one annual global report, a large amount of different dyes, such as acid, reactive, disperse, metal complex, mordant, direct, basic and sulphur dyes, are used for industrial purposes. Among these, azo dyes (having an azo group consisting of two nitrogen atoms (−N=N−) are largely used in the textile and other different industries.1) Reactive Black B (RBB) is widely used for industrial purposes and capable of causinge some serious problems for aquatic and human life. These issues have led to new regulations concerning colored wastewater discharge as well as to the development of more efficient treatment technologies.2) Up to now, these different strategies involve dye removal form from water, i.e. biodegrading, coagulation, the advanced oxidation process and the membrane process.3–7) All the aforementioned techniques have certain advantages and disadvantages vis a vis other techniques. Therefore, convenient techniques are required for the degradation of dye from water. Recently, there has been a focus on semiconductor photo catalysis in the removal of dyes from wastewater, particularly, because of the ability of this method to completely mineralize the target pollutants.8) Heterogeneous photocatalysis involves different fields of science. Physical, chemical, and biological engineering, along with various different approaches such as using photoelectrochemical cells in aqueous or organic solvents, have also been widely investigated for conversion of light to electricity.9) Chemical photosynthesis and fuel production are other important applications of heterogeneous photocatalytic systems.10,11) Various semiconductor materials such as TiO2, ZnO, and CdS have been consistently used for their catalytic degradation to resolve environmental pollution problems. However, the practical applications of TiO2 are limited due to this material’s high recombination rate of photo induced charge carriers and high band gap energy.12–15) Among various semiconductor nanocrystals, those with small band gap such as transition metal dichalcogenide compounds, and MX2 (M = Mn, Fe, Co, Ni; X = S, Se, Te), are interesting for fundamental research due to their unique magnetic and electronic properties. Among these compounds, cobalt sulfide (CoS2) is a favorable candidate for the degradation of pollutants and/or the production of photocatalytic hydrogen, due to its low band gap and high utilization efficiency.16) Similarly, the addition of a carbon nanostructure such as carbon nanotubes or graphene components to a coupled semiconductor may also enhance overall activity. Many strategies have been developed to combined graphene and other photocatalysts in binary or ternary forms.17,18) Graphene plays a crucial role in the photocatalysis filed: graphene-based materials have been broadly studied due to their unique properties (e.g. high thermal conductivity ≈ 5000 Wm−1k−1 and high surface area ≈ 2600 m2g−1). These noteworthy properties enhance the charge transfer separation of the electrons and holes.19,20) These photogenerated holes and electrons play important roles in photocatalytic degradation.
In this paper, we prepare a CoS2-G-TiO2 ternary nano composite through microwave-assisted techniques. By looking at the degradation of industrial dyes (RBB), we also study the photocatalytic activity under irradiation with visible light.
2. Experimental Procedure
2.1. Materials
Cobalt chloride (CoCl2), anhydrous purified sodium sulfite (Na2SO3, 95%), and ethyl alcohol (94%) were purchased from Duksan Pure Chemical Co. Ltd., Korea. Titanium(IV) n-butoxide (TNB, C16H36O4Ti) as the titanium source for the preparation of the CoS2-graphene/TiO2 composites was purchased as reagent-grade from Acros Organics, USA. RBB, purchased from Texchem Korea Co. Ltd, was used as a standard industrial dye. All chemicals were used without further purification. All dilutions were carried out using distilled water.
2.2. Synthesis of CoS2-graphene composite
The graphene nanosheets (50 mg) prepared by Hummer’s-Offeman method were stirred for 30 min then added to CoCl2 and Na2SO3 mixture solutions. The mixture solutions were transferred 500 ml reaction vessels and placed in a conventional microwave for 500 s, with 10 sec on and 10 sec. The solution was then irradiated with microwaves for 500 s, with periodic offsetting after 10 s. The mixtures were then cooled to room temperature, filtered with Whatman filter paper, and heat treated for 5 h at 90°C to obtain a CoS2-graphene powder
2.3. Synthesis of CoS2-G-TiO2 composite
CoS2-G-TiO2 was prepared using pristine concentrations of TNB and CoS2-graphene composites. Both solutions (CoS2-graphene + TNB) were mixed at molar ratio of CoS2-graphene : H2O : TNB = 35 : 15 : 4 and then heated at 60°C for 20 min The obtained solutions were finally transferred to 500 ml reaction vessels and placed in a conventional microwave oven. The solutions were then irradiated with microwaves for 500 s, with periodic offsetting after 10 s. The mixture was then cooled to room temperature, filtered with Whatman filter paper, and heat treated for 5 h at 90°C to obtain a CoS2-G-TiO2 nanocomposite.
2.4. Characterization
XRD (Shimadzu XD-D1, Uki, Kumamoto, Japan) with monochromatic high-intensity CuKa radiation (λ = 1.5406 Å) was used to determine the crystallinity of the composite. SEM (JSM-5600, JEOL Ltd., Tokyo, Japan) was used to examine the surface morphology of the CoS2-G-TiO2 nanocomposite. Transmission electron microscopy (TEM, JEOL, JEM-2010, Japan) was used to investigate the state and particle size of the prepared composite, and Raman spectra of the prepared samples were obtained using a spectrometer (Jasco NRS-3100) with an excitation laser wavelength of 532.06 nm. The photocatalytic performance of the prepared samples was investigated by absorbance spectrometry obtained using a UV/Vis spectrophotometer (Optizen POP, Mecasys, Korea).
2.5. Photocatalytic degradation experiment of Reactive Black B (RBB)
The adsorption and photocatalytic performance of the as-prepared CoS2-G-TiO2 was estimated by the degradation of RBB dye under visible light. A xenon lamp (8 W, λ > 420 nm) was used as a visible light source. In the experiment, 30 mg of the CoS2-G-TiO2 catalytic sample was put in a 100 ml solution of RBB (3.0 × 10−6 mol/L). In order to reach adsorption-desorption equilibrium, the solution was kept in the dark for 30 min. Then, a 10 ml sample was collected from the solution and put in a centrifuge at 10,000 rpm for the elimination of solid materials. After that, the light source was turned on. Samples were collected every 30 min and then centrifuged for 10 min to remove any suspended solid. To compare their catalytic efficiencies, all samples were irradiated for 180 min.
3. Results and Discussion
The structural properties of the fast microwave-assisted technique method synthesized CoS2-G-TiO2 nanocomposite were characterized and compared to those of CoS2 by XRD analysis (Fig. 1). The characteristic peaks of CoS2 at 2θ = 28.10, 32.20, 35.62, 40.30, 47.50, 49.20, 55.21, 61.00, and 63.22° were assigned to the (1 1 1), (2 0 0), (2 1 0), (2 1 1), (2 2 0), (221), (311), (023), and (321) planes of the crystallize structure of CoS2 (space group pa-3(205), a = b = c = 5.535) (JCPDS Card No 00-65-3322).21) The XRD pattern of TiO2 has diffraction peaks around 2θ of 25.5, 38.5, 48.6, 56.5, 68.2 and 71.50, which can be indexed to the characteristic peak (101), (112), (200), (211), (116), and (220) that correspond to the anatase crystal phase (JCPDS PDF#00-021-1272). Furthermore TiO2 (101) and graphene (002) peaks are positioned at the same 2θ values. This demonstrates the successful formation of CoS2-G-TiO2 ternary nanocomposite.
To investigate the morphological structure of CoS2-G-TiO2 we use scanning electron microscopy (SEM). Fig. 2 shows that the CoS2-G-TiO2 sample had a plate like morphology of graphene; the images clearly show graphene as a sheet-like structure broken off in different directions. In Fig. 2(a), the CoS2 particles are the main particles and are uniformly dispersed on the graphene sheet. Fig. 2(b) indicates that the TiO2 was ruggedly distributed on the graphene sheet. In Fig. 2(c), it can be seen that the TiO2 and CoS2 are uniformly mixed on the graphene sheet, leading to an increase of the nanoparticle size. CoS2 nanoparticles were of almost spherical shape with partial agglomeration. After microwave treatment, the sheet morphology is maintained and the graphene surfaces are covered with CoS2 and TiO2 nanoparticles. The plate-like structure of graphene shows the existence of oxygen functionalities on the surface of the graphene. The attachment of nanoparticles helps to overcome the interactions between functionalities on the graphene surface.
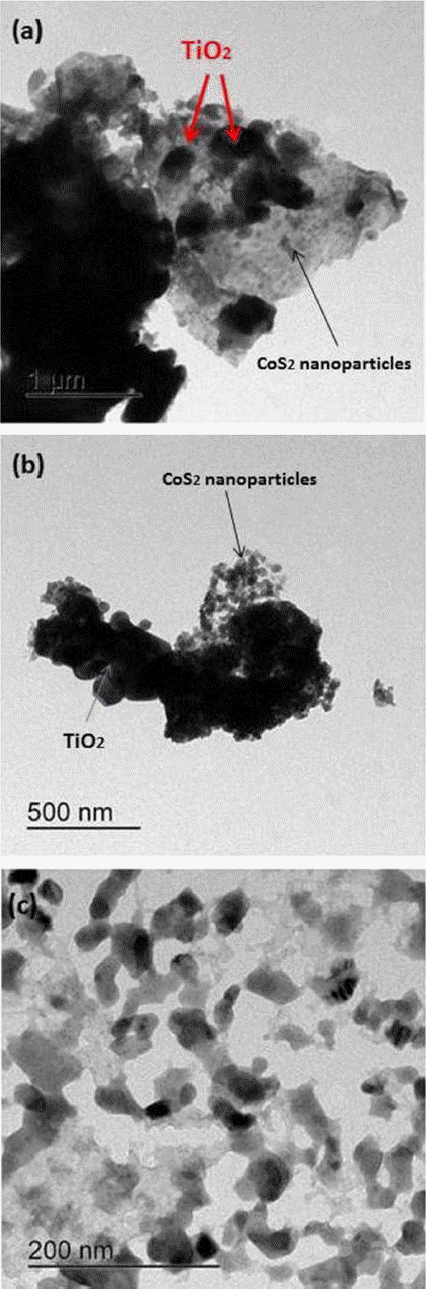
For the microscopic structural information we used TEM. Fig. 3(a–c) displays different magnification images of the CoS2-G-TiO2 nanocomposite, Fig. 3 reveals that graphene, consisted of thin stacked flakes and had a well-defined few-layer structure at the edge, while CoS2 and TiO2 nanoparticles were mixed on the graphene sheet in the form of bunches of clusters. The irregular light and dark images shown in Figs. 3(a–c) reveal that CoS2 was partial agglomerated, while TiO2 nanoparticles were evenly dispersed on the graphene nano-sheet. Hence, the CoS2 nanoparticles support the TiO2 nanoparticles on the graphene sheet and provide a bridge between TiO2 and the graphene nanosheet; we thus assume that a microwave assist technique is favorable for enhancing the photocatalytic properties of the nanocomposite. This provides clear evidence of the good contact and interaction among TiO2, CoSe2 nanoparticles and graphene.
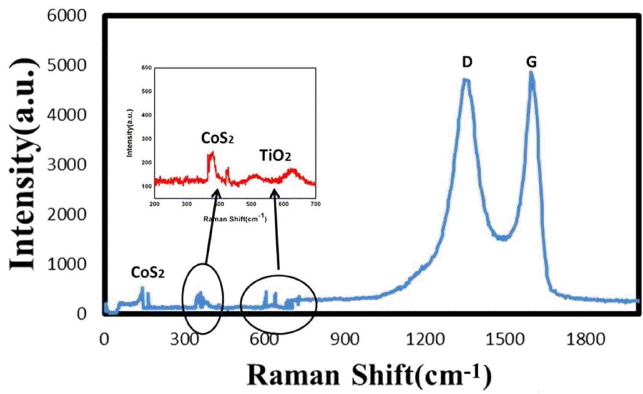
Figure 4 shows the Raman spectra of the CoS2-G-TiO2 nanocomposite, with both typical D and G bands of graphene. Fig. 4 shows two prominent peaks, corresponding to the G band located at 1583 cm−1 and the D band located at about 1353 cm−1. These G and D bands show the molecular picture of the carbon material. The D band corresponds to sp3 bonding in response to defects,22–24) The G band is associated with the doubly degenerate (iTO and LO) phonon mode (E2g symmetry) at the Brillouin zone center.24) In fact, the G-band is the only band in graphene coming from a normal first order Raman scattering process. The CoS2 nanoparticle characteristic peaks are at 220, 389, and 410 cm−1. These results are in close agreement with a report for CoS2 single crystals.25) TiO2 anatase peaks were observed, to be located at 510 and 640 cm−1 corresponding to the A1g + B1g(2), and Eg(2) modes.26,27) Moreover, ID/IG ≈ 1.002, indicates the increase in the number of graphene layers or the partial reduction of graphene oxides into graphene.28)
The photocatalytic performance of the CoS2-G-TiO2 nanocomposites in terms of photo degradation of RBB under visible light irradiation was investigated. Fig. 5(a–e) shows the UV-visible absorption spectra of the RBB solution after visible light irradiation of different intervals of time using the TiO2-G, CoS2-G, and CoS2-G-TiO2 nanocomposites. Due to visible light irradiation, the dye concentration constantly decreased with the passage of time. The two main steps involved in the photocatalytic decomposition of the dyes were, (1) the adsorption of dye molecules and (2) the degradation of the dye molecules.29,30) After corrective adsorption in the dark for 30 min with magnetic stirring, the samples reached adsorption-desorption equilibrium, and different composites showed different physical adsorption effects, Fig. 5(a–c) shows that after irradiation of 180 min, the CoS2-G, TiO2-G, and CoS2-G-TiO2 nanocomposites were degraded by 53%, 37%, and 89% respectively. As expected, the ternary nanocomposite had outstanding performance, better than those of the CoS2-G and TiO2-G nanocomposites. The decrease in the concentration and the excellent catalytic effect can be attributed to the homogenous distribution of CoS2 and TiO2 on the graphene surface. The metallic components of the graphene sheet generate a Schottky barrier at the interface with TiO2 and the graphene sheet, and hence trapped electrons will transfer and separate the excited molecules absorbed at the interface of the CoS2 and the graphene sheets by retaining the recombination process.31) This may lead to the possibility of enhancing the photocatalytic degradation efficiency. Kinetic studies have observed the basis of the degradation rate of the dye. This is formulated by the Langmuir-Hinshelwood model, as shown in Fig. 5(e) and calculated using the following equation.32)
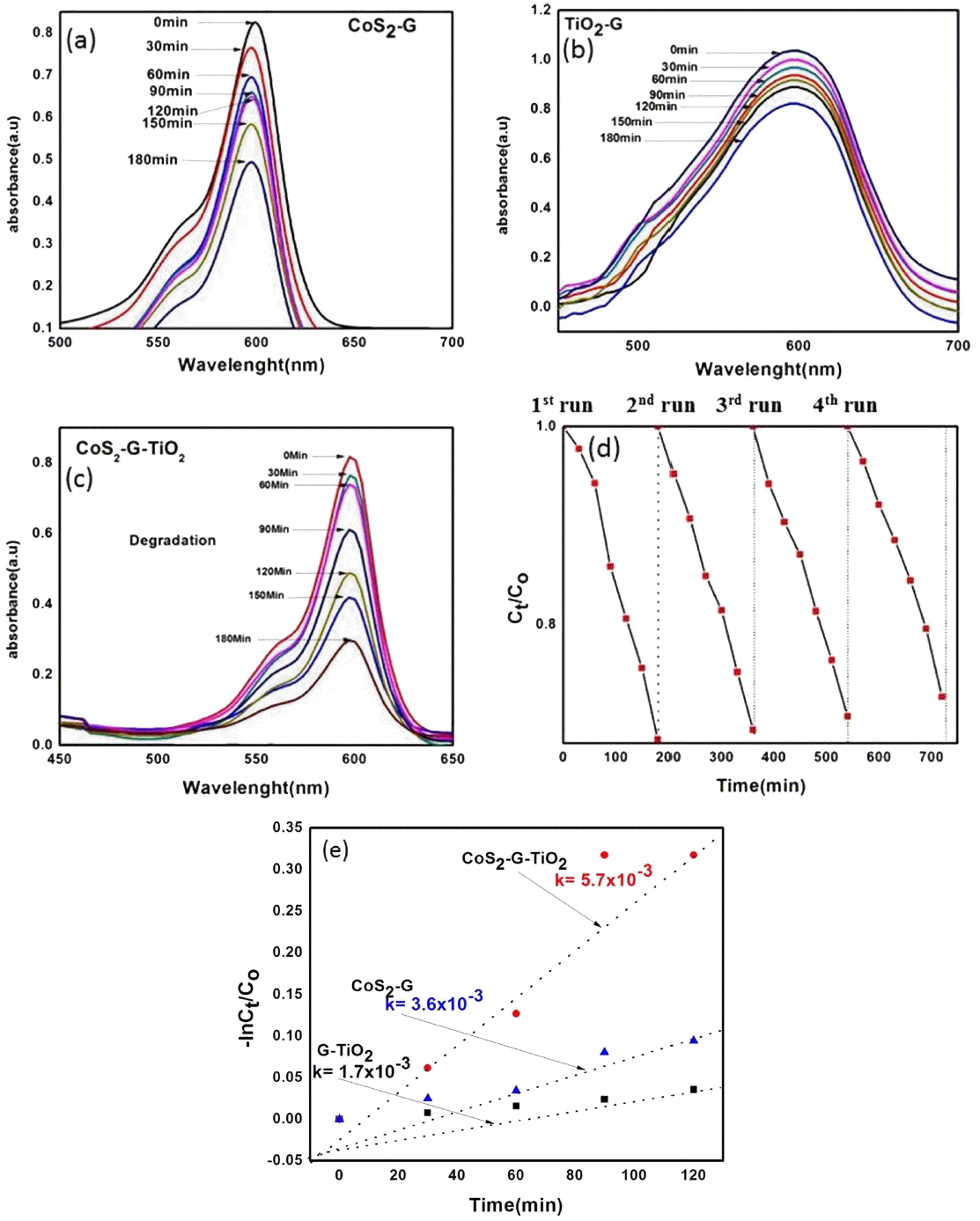
UV-vis diffuse reflectance spectra of (a) CoS2-G, (b) TiO2-G, and (c) CoS2-G-TiO2, (d) Recyclability of CoS2-G-TiO2 nanocomposite and (e) kinetics of degradation of prepared composites under visible light irradiation.
where Co = C = initial concentration and K = Pseudo 1st order kinetic constant.
In the graphical plot in Fig. 5(e), the slope of the linear plots should be equal to the apparent first order rate constant (Kapp). Fig. 5(e) shows that the RBB degradation constant rate of the CoS2-G-TiO2 nanocomposite was higher than those of TiO2-G and CoS2-G. These results further reveal that the CoS2-G-TiO2 ternary nanocomposite is more efficient than the binary nanocomposites, i.e. CoS2-G, or TiO2-G.
Figure 5(d) shows the results of cyclic experiments for RBB as organic dye under micro wave assist technique. From the results, it can be clearly observed that the CoS2-G-TiO2 nanocomposite did not show any significant loss of photocatalytic activity after four runs of RBB degradation; these results indicate the great stability of the nanocomposite. Due to their high stability and excellent photocatalytic efficiency, CoS2-G-TiO2 nanocomposites are an emerging candidate for environmental remediation.
4. Conclusions
In present work, we successfully synthesized a CoS2-G-TiO2 ternary nanocomposite by a facile microwave-method. SEM and TEM images clearly show that CoS2 was uniformly distributed on graphene sheets supported by TiO2 nanoparticles, XRD and Raman spectra results show a significant reduction of GO to rGO and also confirm the presence of CoS2, and TiO2 anatase crystal phase. The photocatalytic performances of CoS2-G-TiO2, CoS2-G, and TiO2-G were evaluated using reactive black B (RBB). It is clearly revealed that the CoS2-G-TiO2 ternary nanocomposite can be used as a competent photocatalyst under visible light irradiation. This excellent photocatalytic activity is due to the positive synergistic effects of CoS2, TiO2, and graphene. The current study has provided a new route for graphene based materials to be be used for environmental remediation.