Effect of the Si-C Powder Prepared by Mechanical Alloying on the Densification of Silicon Carbide Powder
Article information
Abstract
High purity Si-C (99.999%) powder prepared by mechanical alloying was added to a commercial SiC powder as a sintering additive. Reaction bonded silicon carbide balls and jars with high purity (99.98%) were used for the mechanical alloying. As a result, the purity of the sintered Si-C was higher than 99.99%. When sintered at 2200°C under 50 MPa pressure for 1 h, SiC containing 10 wt% of high purity Si-C showed a relative density of 95.3%, similar to the relative density of commercial SiC (95%). However, the relative density of SiC decreased to 90.6% without the additive when the applied pressure decreased to 40 MPa. In contrast, the relative density was nearly unaffected by the decrease of the pressure when using the Si-C additive. Therefore, the addition of Si-C powder promoted the densification of SiC above 2000°C under 40 MPa pressure.
1. Introduction
Recently, integration of semiconductors and refinement of linewidths has been rapidly implemented, according to which semiconductor process conditions of wafers are being more strictly controlled than in the past. Also, as the generations of electronic products are developed, jigs of high strength and high elastic modulus for semiconductor process are required for larger-scale, larger-area products. Consequently, development of new materials is being required to substitute for the quartz glass ceramic jigs for semiconductor process which have been widely used in the past.
Since dense, high-purity SiC not only has excellent thermal and mechanical characteristics but also satisfies diversified properties required for semiconductor processes such as excellent plasma-resistant properties and low metal ion contents, etc., it is noted as a material which can minimize inflow of impurities into semiconductors during the semiconductor processes.1) Accordingly, while SiC products with excellent mechanical, thermal, and chemical characteristics having a purity equivalent to or higher than quartz glass products employed in the past are being produced by reaction sintering method, CVD method and recrystallization method, these products have shortcomings such as property degradation due to residual Si, high prices along with strength reduction due to a low relative density.2–4)
Recently, ultrahigh-purity SiC products are being produced by sintering method, which have a lower cost than CVD-SiC while showing similar purities and mechanical characteristics as those of the products prepared by CVD method. In addition, the materials exhibit excellent corrosion resistance, plasma resistance and mechanical characteristics compared with the products manufactured by reaction sintering method and recrystallization method. Using the ultrahigh-purity SiC prepared by sintering method, the products such as Dummy wafer and heater have been commercialized, and applicable items are being expanded to sputtering target, diversified electrical components as well as sensors.5)
However, because SiC is difficult to sinter due to highly covalent bonds and low self-diffusion coefficients, pressure sintering under a high pressure around 40 MPa at a high temperature above 2300°C must be generally performed for a long time to achieve densification of high-purity SiC powders, which becomes a factor imposing constraints to size and price of the products.
As a method for promoting sinterability of SiC without the addition of sintering aids, the method of using Si-C powder of fine particle size which is obtained by high-energy milling of Si and C as the starting materials has been reported. Yamamoto et al. have compared densification behaviors of SiC powder obtained by Mechanical alloying method(to be referred to as MA hereafter) and of commercial nano-SiC (d50: 30 nm) powder, and reported that the relative density of MA-SiC was increased to 98% by drastic densification above 1650°C whereas the relative density of commercial nano-SiC was less than 60% upon sintering by using a Spark plasma sintering furnace at 1700°C under a pressure of 40 MPa for 10 minutes.6) They reported that densification was induced without large grain growth because dis-ordering such as amorphous structure and stacking fault inside MA-SiC was rearranged above 1650°C.
However, since impurity contamination from the milling media, such as ZrO2, Si3N4 ball or chrome steel, could not be prevented in the existing studies, high-purity SiC sintered bodies could not be prepared by using this method. Recently, however, commercial SiC having a purity of 99.98% prepared by reaction bonded silicon carbide (to be referred to as RBSC hereafter) has been developed, through the use of which balls and jars for milling can be manufactured.
Consequently, when the Si-C powder obtained by mechanical alloying using the high-purity RBSC milling media is used to promote densification of the existing high-purity commercial powders, the sinterability can be expected to be improved without sacrificing the purity. In the present study, the effects of SiC on sintering behavior have been investigated when a small amount of Si-C powder prepared by mechanical alloying method using the high-purity RBSC milling media is added to the existing high-purity commercial powder.
2. Experimental Procedure
As raw material for the experiments, high-purity Si powder of 5N-class (high purity, 99.999%, 75 micrometer pass) and graphite powder (Alfa aesar, 99.9995%, 325 mesh pass) were used by mixing in the molar ratio for ball vs. powder of 1 : 1. Weight ratio for ball vs. powder was 5 : 1, with the amount of powder subjected to milling once being fixed at 10 g.
As high-purity RBSC containers and balls (ϕ10 mm) for the experiments, commercial products of 99.98% purity were used (3.8N grade, Inocera, Korea, residual Si content: 24%). Milling was performed in vacuum (10− 2 Pa) by dry method at 400 r.p.m. using a planetary mill (Fritsch, Pulverisette 5) with the ratio for ball : powder being fixed at 5 : 1, and the holding time at 72 h.
For commercial SiC powder (LG Innotek, 99.999+%, d50: 0.7 micrometer) and Si-C powder synthesized by Mechanical alloying (to be referred to as MA-SiC hereafter), phase-forming behavior along with changes in particle size and shape were observed by using XRD (D/MAX 2200, Rigaku, Tokyo, Japan), a particle size analyzer (Beckman Coulter, LS I3 320), SEM (JSM 5800, Jeol, Tokyo, Japan) and TEM (JEM 2100F, JEOL, Tokyo, Japan). Oxygen contents in the powder were analyzed by using an oxygen-nitrogen analyzer (ON900, Eltra).
Subsequently, after mixing 10 wt% of MA-SiC powder in commercial SiC powder it was mixed by dry method in vacuum at 200 r.p.m by using a planetary mill. To find the effects of dry milling on the characteristics of the commercial powder, some commercial powder without the addition of MA-SiC was subjected to dry milling under the same conditions.
Sintering of the raw material powder was conducted at a heating rate of 50°C/min. under Ar atmosphere using a spark plasma sintering (SPS, Dr. Sinter 4000, SPS Syntax, Japan) furnace. Sintering temperature for Si-C was fixed at 2200°C while sintering pressures were varied between 40–50 MPa, and holding times between 30 min – 1 h. Sintered bodies of ϕ20 × 3 mm in a pellet form were prepared by using 4g of powder for each.
After sintering, relative densities were measured by using Archimedes principle, and purities of the sintered body measured by using glow discharge mass spectrometry (GD-MS, Element GD, Thermoscientific, MA, USA).
3. Results and Discussion
Figure 1 shows the morphologies of commercial high-purity SiC powder, MA-SiC powder and the commercial powder subjected to 12 h of dry milling after the addition of 10 wt% of MA-SiC. According to SEM observation results, a bimodal form of particle size distribution was exhibited where relatively coarse particles having a size larger than 1 micrometer and nano particulates of less than 100 nm were mixed (Fig. 1(a)). In particular, very fine powders around 20–30 nm in size were also present. When these powders were milled at 200 r.p.m. for 12 h by using high purity RBSC milling media under the condition of ball : powder ratio = 5 : 1, fine particles with soft agglomeration was observed (Fig. 1(b)). While MA-SiC powders around 50–150 nm were observed, these powders were the agglomerates of a mixture of amorphous Si and C as well as fine b-SiC of 3–10 nm in size formed by mechanical alloying (Fig. 1(c), (d)). According to the particle size analysis results, the synthesized powder was very fine with an average particle diameter of 170 nm (Fig. 1 (e)). In the powder mixed at 200 r.p.m. for 12h after the addition of 10 wt% of MA-SiC to commercial SiC, coarse powders and fine powders around 100 nm were mixed, and a smaller amount of fine powders having a size of 20–30 nm as compared with that of commercial SiC were observed, which is attributed to the fact that the fine powders formed agglomerates with MA-SiC during mixing (Fig. 1 (f)).
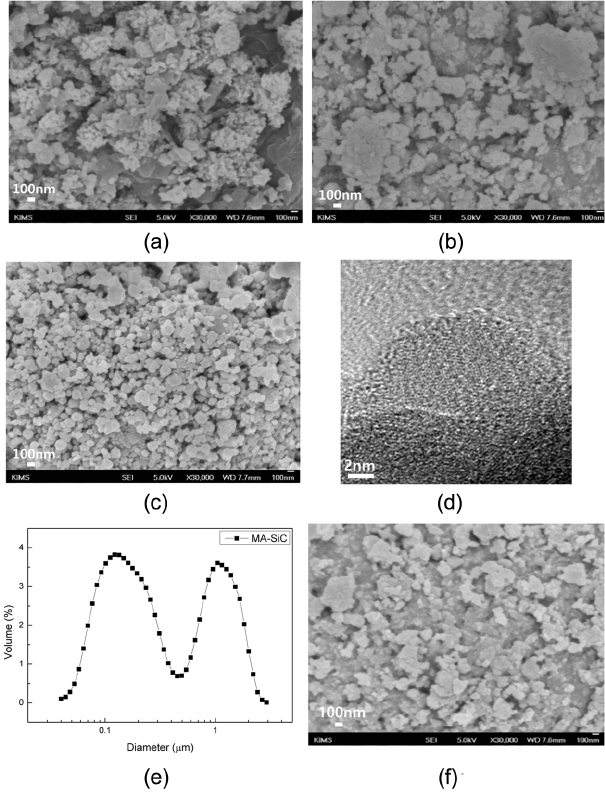
Morphology of the powders before and after milling. (a) commercially available SiC, (b) commercially available SiC, after milling for 12 h, (c) MA-SiC, (d) TEM image of MA-SiC, (e) particle size distribution of MA-SiC and (f) commercially available SiC + 10 wt% MA-SiC after milling for 12 h.
Table 1 shows oxygen contents for the powders processed under various conditions. Considering the possibility of oxidation for nano SiC powder which is present within the powder, the commercial SiC powder exhibited a relatively low content of residual oxygen (2.63%). The oxygen content was increased by about 1 wt% after 12 h milling, which is considered attributable to the fact that oxygen and moisture penetrated the jar interior during milling to cause oxidation of the raw material powder and additional oxidation also occurred during the processes such as powder sieving, etc. after milling. In the case of MA-SiC, the oxygen content was 2.76%. Although the residual oxygen content for coarse-grained (75 μm pass) Si raw material powder used for the experiments was low (0.5 wt%), about 2 wt% of oxygen have been additionally included because of oxidation due to fine particle sizes during and after milling. Consequently, the oxygen content in the powder was shown to be the highest in the case of 12 h milling after mixing 10 wt% of MA-SiC with SiC.
Figure 2 shows XRD patterns for raw material powders before and after mixing under the condition of 200 r.p.m. and 12 h. In the case of commercial powder, single-phase b-SiC was detected. A weak Si peak appeared around 28 degrees upon 12 h milling although no phase changes could be observed, which is due to contamination of RBSC milling media by some free Si during milling (Fig. 2 (a),(c), residual Si content in RBSC: 24%). When 10 wt% of MA-SiC was mixed, the Si peak intensity was shown to be larger than when only commercial SiC was milled, which is due to the presence of residual Si in MA-SiC in addition to contamination from RBSC (Fig. 2 (b), (d)). Si has a low contact angle with SiC upon melting at a high temperature, with excess Si known to promote liquid-phase sintering by being collected at SiC grain boundaries.7,8) In the present study, although most of residual Si in MA-SiC is converted to SiC by the reaction with carbon upon temperature rise, Si contaminated from RBSC milling media is considered capable of affecting densification. However, the effects were observed to be slight in the present study, which will be discussed later.

XRD patterns of the powders before and after milling: (a) commercially available SiC, (b) SiC + 10 wt% MA-SiC after milling for 12 h, (c) commercially available SiC after milling for 12 h, and (d) MA-SiC (■: graphite, □: SiC, ●: Si).
Figure 3 shows the displacement data during sintering at 2200°C under 50 MPa pressure using the powders processed under various conditions. The shrinkage behavior of powders during SPS was measured by the displacement of the lower electrode of equipment, where not only linear expansion of the powder but also the displacements of graphite punch for the mold containing powder were also measured. Therefore, at temperatures below 1300°C where no shrinkage of powders occurred, thermal expansion behavior, due to heating of the raw material powder, the graphite punch and the graphite spacer, was observed as a result of temperature increase.
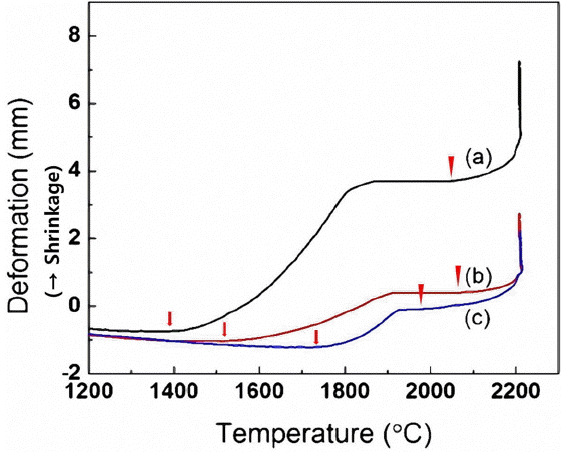
Shrinkage behavior of various powders sintered at 2200°C under 50 MPa pressure in Ar: (a) commercially available SiC, sintered for 1 h (b) commercially available SiC after milling, sintered for 1 h and (c) SiC + 10 wt% MA-SiC after milling, sintered for 30 min. (↓: Onset of 1st shrinkage, ▼: Onset of 2nd shrinkage)
All powders employed in the experiments exhibited 2-step shrinkage behavior. In the case of commercial SiC power, the 1st shrinkage occurred at 1390 – 1870°C. Due to the strong covalent bond character, SiC did not show active occurrence of densification at temperatures below 1900°C when no sintering aids were added. Nadeau et al. reported that an ultra-high pressure around 2 GPa and a high temperature above 2100°C were required for the densification of high-purity SiC.9) Therefore, the 1st shrinkage is considered to have occurred by particle rearrangement due to sliding, etc. Relative density of the specimens sintered at 1800°C under a pressure of 40 MPa for 30min was 69% indicating that densification did not strongly occur, and almost no sintering shrinkage was observed while being held at the sintering temperature. Based on this observation, the 1st shrinkage is attributed mostly to the improvement in packing ability of the powder by particle movement, etc. caused by the softening of SiO2 which presents on the SiC surfaces under a high pressure (40 MPa). The 2nd shrinkage was started from 2065°C. Under the condition of 2200°C and 50 MPa, the relative density after sintering for 1 h was 95.9%, indicating that relatively dense sintered bodies could be obtained.
When commercial SiC powder was milled for 12 h, the 1st shrinkage occurred at 1510 – 1910°C. For the powder subjected to milling processing, deformation by the 1st shrinkage was reduced in comparison with the powder before processing. Based on this observation, it was considered that particle packing behavior was affected by milling and the milled powder had a higher packing ratio than the original powder upon initial packing. The 2nd shrinkage by sintering occurred above 2070°C, and the 2nd shrinkage temperature was not greatly affected by milling. The relative density after the sintering for 1 h was 95%. The relative density decreased slightly for the milling-processed powder compared with the as-received powder, which is attributable to the fact that SiO2, which formed during milling, was decomposed at a high temperature. Based on this result, it could also be seen that a small amount of Si intermingled from RBSC media during milling did not strongly promote densification.
In the case of commercial SiC + 10 wt% MA-SiC powder, the 1st shrinkage was further reduced and occurred at 1730 – 1925°C. This is considered to be caused by an increase in the initial green density by the effective filling of voids when powders with different particle size ranges were mixed. Actual relative densities for commercial powder, milled commercial powder and commercial powder with the addition of 10 wt% of MA-SiC after cold isostatic pressing(CIP) for 5 min under 200 MPa are given in Table 2. The relative density of the green body could be seen to be increased progressively after milling and upon the addition of MA-SiC. However, the 2nd shrinkage temperature was markedly reduced so that the sintering shrinkage occurred above 1970°C, based on which sintering could be seen to have been started at a temperature lowered by 100°C in comparison with the case without addition of MA-SiC. This is attributed to the fact that initial sintering of MA-SiC having the excellent sintering characteristics was started at a lower temperature compared with that of commercial powder.6)
Also, above 2070°C where sintering shrinkage of the milled commercial SiC was started, faster sintering shrinkage occurred due to addition of MA-SiC. Consequently, the relative density after sintering for 30min under the condition of 2200°C and 50 MPa was 95.34%, showing a higher relative density than that of the commercial SiC milled and sintered for 1 h.
Figure 4 shows sintering shrinkage behavior when the milled commercial SiC powder and the powder containing 10 wt% MA-SiC were sintered at 2200°C under 40 and 50 MPa. When the pressure was lowered to 40 MPa, the commercial SiC had a relative density of 90.6% after sintering for 50 min, indicating that densification did not properly occur. Whereas the 1st shrinkage temperature for the commercial powder was 1395°C which was similar to that for the case of 50 MPa, the 2nd shrinkage temperature clearly increased to 2140°C. In contrast, when MA-SiC was added, sintering shrinkage behavior similar to the case under 50 MPa pressure was observed, with a relatively high relative density of 95.3% measured after sintering for 50min. Based on such results, it could be seen that MA-SiC promoted the sintering of commercial SiC powder where the sintering promotion effects were more clearly shown, particularly when the pressure was lower than 40 MPa. Yamamoto et al. have reported that clear densification to a relative density of 98% occurred upon application of a pressure of 40 MPa in the case of MA-SiC, whereas obvious densification was not observed at 1700°C when pressures less than 40 MPa were applied. They reported that low-temperature densification of MA-SiC was induced by dis-ordering such as amorphous structure and stacking fault, etc. inside the powder, which began to be actively rearranged under the condition around 1700°C and 40 MPa.6) In the present study, densification was promoted for commercial SiC under a pressure higher than 50 MPa whereas clear sintering promotion was also observed for MA-SiC under a pressure of 40 MPa, which is attributed to the fact that the pressure required for densification by rearrangement of disordering present inside MA-SiC was lower than the pressure required for densification of the crystalline SiC.

Sintering behavior of various powders in Ar. (a) commercially available SiC after milling, sintered at 2200°C under 40 MPa pressure for 1 h, (b) commercially available SiC + 10 wt% MA-SiC, sintered at 2200°C under 40 MPa pressure for 50 min, (c) commercially available SiC + 10 wt% MA-SiC, sintered at 2200°C under 50 MPa for 30 min.
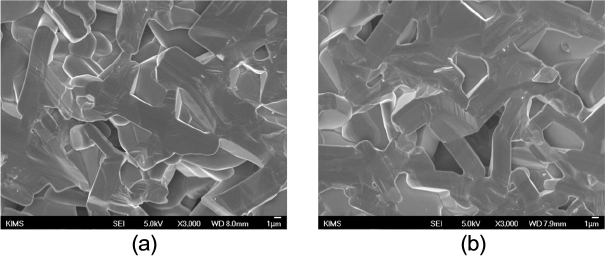
In the specimens with sintering completed at 2200°C, clear grain growth occurred (Fig. 5). This was commonly observed for both the cases without the addition of activating SiC and with the addition of 10 wt%, where plate-like grains of around 5 – 10 μm were observed (Fig. 6). Based on this observation, it could be concluded that densification by matter movement took place during the 2nd shrinkage and that densification and grain growth by matter movement actively occurred at the sintering temperature of 2200°C.
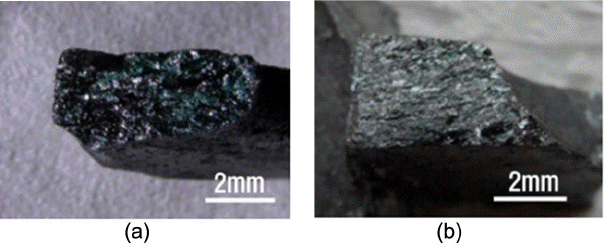
Fractured surface of the specimens sintered at 2200°C under 35 MPa for 30 min in Ar (a) commercially available SiC, (b) commercially available SiC + 10 wt% MA-SiC.
4. Conclusions
High-purity Si-C powder prepared by mechanical alloying method using high-purity reaction bonded SiC milling balls and jars with the purity of 99.98% promoted the sintering of high-purity commercial SiC with a purity higher than 99.999%. The sintering promotion effects of the Si-C powder were prominently shown for the case where the sintering pressure was lower than 40MPa. The addition of Si-C promoted densification below 1950°C through the improvement of particle packing due to a particle size difference, and the temperature of densification by matter movement also decreased by 100°C from 2070°C to 1970°C. In addition to an increase in packing density by the addition of fine powders, such lowering of densification temperatures is considered to be induced through the rearrangement of dis-ordering such as amorphous structure and stacking fault, etc. inside the Si-C powder which was formed during mechanical alloying process.