1. Introduction
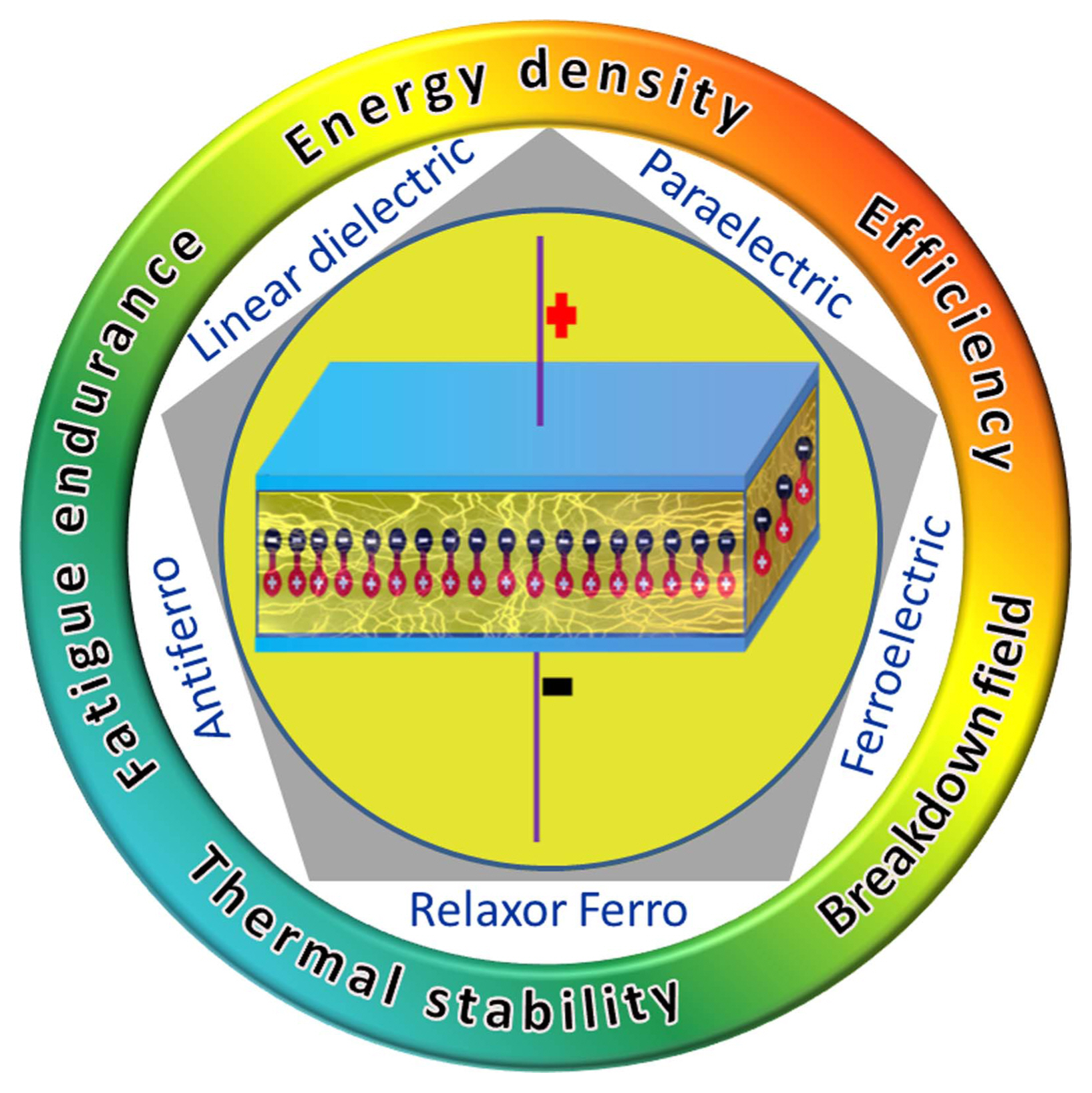
Capacitors are widely employed as passive components in many electronic devices. They are used for carrying out a host of functions such as pulse discharging, filtering, voltage smoothing, coupling, de-coupling, dc blocking, power conditioning, snubbing, electromagnetic interference suppression, and commutation in pulsed power and power electronics applications.1) The technology of capacitors as storage media for electrical energy dates back to the beginning of the 20th century. Through the various stages of development, different materials such as glass, paper, mica, lacquer, polymers, and ceramics have been utilized as capacitor elements.2) In a dielectric capacitor, the insulating material placed between two parallel conductive plates becomes polarized and stores electrical charge in proportion to the electric potential between its terminals (Fig. 1).
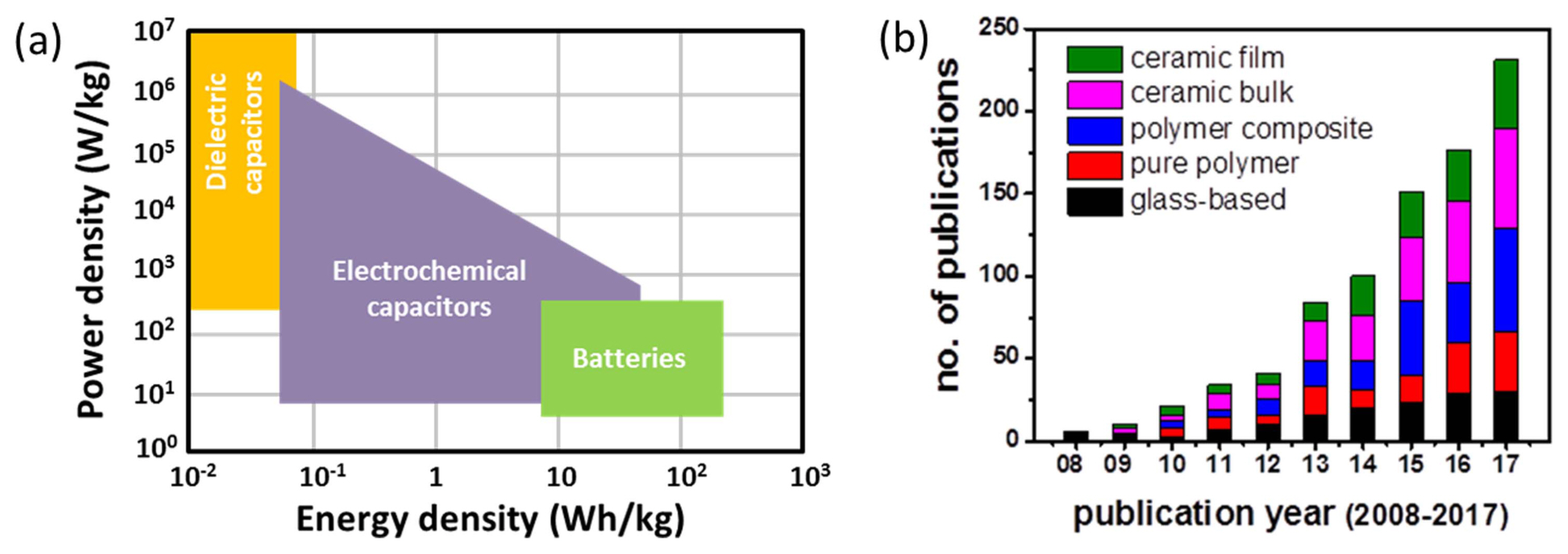
In the past two decades, lots of efforts have been made towards the development of energy storage technologies such as batteries, electrochemical capacitors, and dielectric capacitors to meet the requirement of on-demand utilization of the electricity generated from renewable energy sources. As indicated by the Ragone plot shown in Fig. 2(a), there are significant differences in the energy density and power density of these storage devices, which are attributed to the differences in their energy storage mechanisms and charge-discharge processes. In contrast to the other storage devices, dielectric capacitors can release the stored energy in an extremely short period of time (microseconds-milliseconds) and generate an intensely pulsed current or voltage, which make them suitable for applications in pulsed power electronic systems. Further, unlike the cases of electrochemical capacitors and batteries with liquid electrolytes and involving chemical reactions, dielectric capacitors exhibit superior thermal and mechanical stability and can be operated under higher voltages (several hundred to thousand volts) for longer durations.
In view of their potential applications in pulsed power electronics for use in various commercial, civilian, and military systems, as well as considering the significantly increasing number of publications on dielectric capacitors for energy storage applications in recent years (Fig. 2(b)), this area of research is currently one of the hot topics in the domain of energy storage technologies. The applications of dielectric capacitor-based pulsed power electronics include medical equipment (defibrillators, pacemakers, surgical lasers, X-ray units), scientific research (nuclear effects simulations, high-power accelerators, high-intensity magnetic field experiments), commercial systems (camera flash, food sterilization, metal forming, cable fault detection equipment, underground oil and gas exploration), energy systems (grid-connected photovoltaics and wind turbine generators, power grid fluctuation suppression, high-frequency inverters), transportation (hybrid electric vehicles, electric trains, electric aircrafts), avionics (space-shuttle power systems, rocket propulsion systems), military (active armors, electrochemical guns, radars, high-power microwave devices, ballistic missiles), etc.
Although dielectric capacitors possess high power densities with fast discharge rates, their energy density should be further increased to reduce the volume of the capacitors. Currently, linear dielectric polymer materials with low dielectric constants but high breakdown fields are employed in commercial pulsed power capacitors, exhibiting energy density < 5 J/cm3. To be competitive with supercapacitors, the dielectric capacitors should offer energy density > 30 J/cm3.3) Hence, much of the research on emerging dielectric materials is being carried out in pursuit of enhanced energy density, which would make the dielectric capacitors useful for an even wider variety of applications. The energy storage performance of these capacitors is evaluated in terms of energy density and storage efficiency, which are related to permittivity (ɛ) and polarization (P) of the dielectric material and the external applied field (E) (Fig. 1). For linear dielectrics with negligible loss, the energy density (U) can be expressed as
U = 1 2 ɛ 0 ɛ r E 2
where Pr and Pmax are the remnant and maximum polarizations, respectively. The energy loss density (Uloss) is equal to the hysteresis loss, i.e., the area of the P-E loop. Accordingly, the energy storage efficiency of the capacitor is represented by the ratio between Urec and Ust as follows:
It can be concluded from the above equations that high values of permittivity, maximum (saturation) polarization, and breakdown strength (BDS) will lead to larger energy densities, while low dielectric/hysteresis losses and low remnant polarizations will facilitate greater energy storage efficiencies of dielectric materials. In addition, the material should possess low electronic/ionic conductivity for sustaining high electric fields. However, attaining all these desired qualities in a single dielectric material is a challenging task. An increase in the dielectric constant is often accompanied by an increase in the dielectric loss, leading to thermal management problems. On the other hand, an increase in the applied field stress can lead to early failure and low reliability of the capacitors.4) For maintaining the physical integrity, effective thermal management, and reliable operation of dielectric capacitors over a long time period, high electric fatigue endurance (> 106 cycles) and good thermal stability (−90°C to 250°C) of the dielectric material are essential (Fig. 1). Through a better understanding of the physical phenomena governing the properties of the dielectrics, efforts can be directed to realize an optimum combination of the aforementioned criteria.
To achieve high energy storage performances, various dielectric materials based on polymers, glasses, and ceramics have been studied. Polymer dielectrics exhibit high breakdown field (> 7 MV/cm), but low dielectric permittivity (< 10) and poor thermal stability (< 100°C). In order to improve their permittivity, recently, several polymer-based composites dispersed with dielectric ceramic fillers in different shapes and quantities have been developed.5) Glass-based oxide dielectrics possess greater permittivities and larger breakdown fields (> 10 MV/cm) compared to those of polymer dielectrics. Dielectric ceramics in bulk form exhibit permittivity in the range 10-2000 and their breakdown fields are of the order of 0.1-1 MV/cm. Depending on their dipolar/domain structures and electric-field-dependent changes in the polarization behavior, dielectric materials can be categorized into five major classes, namely, linear dielectrics (LD), paraelectrics (PE), ferroelectrics (FE), relaxor ferroelectrics (RFE), and antiferroelectrics (AFE) (Fig. 3). Each of these dielectric materials has its own advantages and limitations with regard to energy storage capability.
Although many review papers have been published on the dielectric capacitors based on polymers and their composites, reports dedicated to dielectric ceramics are rare.2,3,5,7-13) Recently, Yao et al.3) reviewed the energy storage performances of homogeneous/inhomogeneous-structured dielectrics of polymers, glasses, and ceramics. Chauhan et al.13) presented a review on anti-ferroelectric ceramics for energy storage capacitors. The present review covers most of the literature on dielectric ceramics and provides a comprehensive overview of the studies on the energy storage properties of the bulk ceramics belonging to LD, PE, FE, RFE, and AFE.
2. Dielectric Ceramics for Energy Storage Capacitors
2.1. Linear dielectrics
LD offer high BDS and linear polarization response to an applied electric field with minimal dielectric loss, which allows for large recoverable energy densities and efficiencies. Therefore, various LD ceramic compositions have been investigated for achieving high energy storage performance; some of the compositions are listed in Table 1. It is well known that the energy storage density of the linear dielectric is linearly proportional to the permittivity of the medium and the square of the maximum applied field. A larger BDS contributes more to achieving a high energy storage density. Since the BDS is mainly affected by the phase purity and microstructural features of the ceramics, many efforts have been made to improve it by using suitable dopants/oxide additives,14-18) low melting glasses,19) novel sintering technologies,20) utilizing the core-shell structures of initial particles,21) etc.
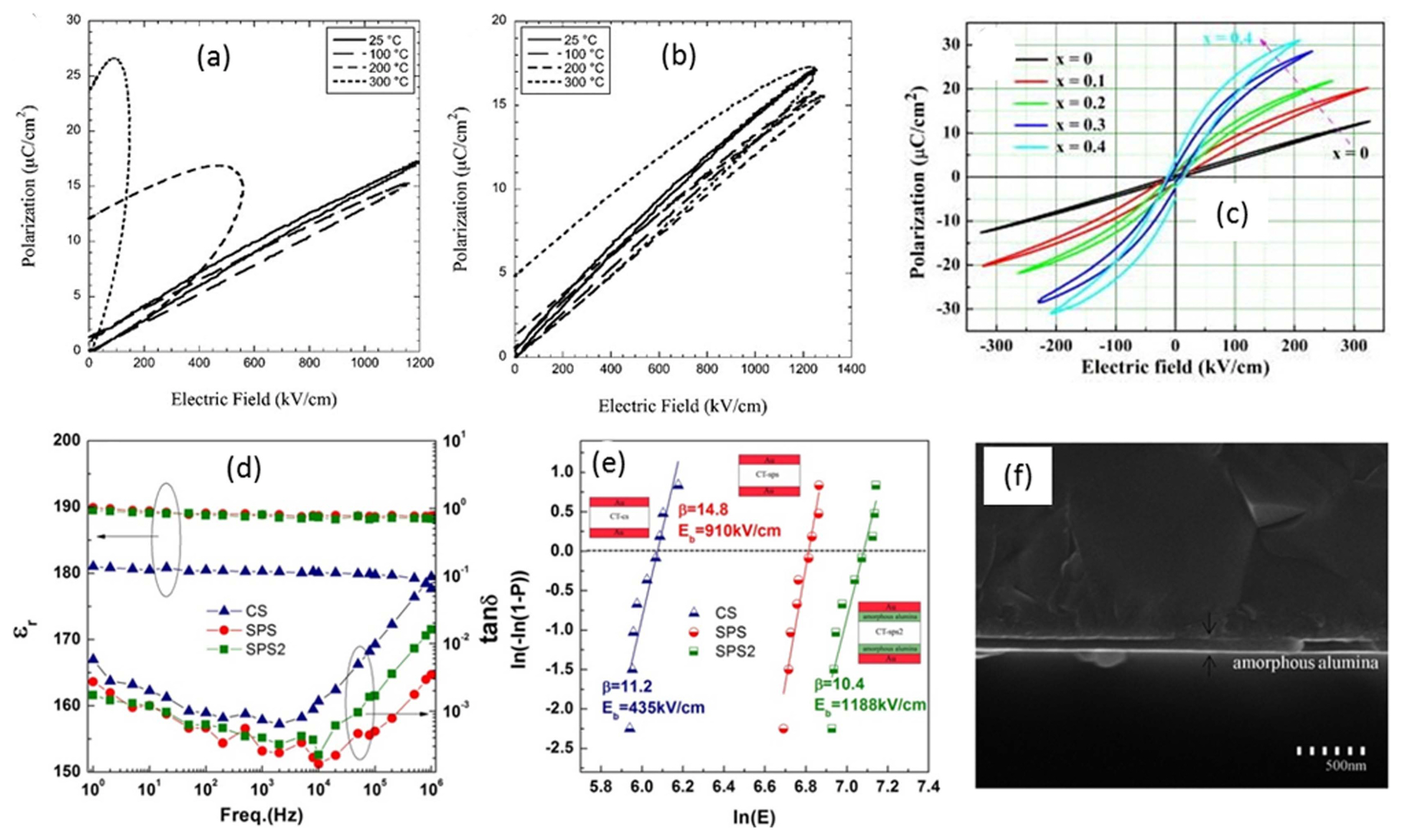
Shay et al.14) investigated the energy storage properties of pure and Mn-doped 0.8CaTiO3-0.2CaHfO3 (CHT) capacitors fabricated via tape casting. For the evaluation of the high electric field behavior of the CHT ceramics, single layers of CHT capacitors (thickness of 9 μm) consisting of Pt internal electrodes were fabricated. Pure CHT ceramics displayed a large energy density of 9 J/cm3 at 1200 kV/cm at room temperature (RT) owing to their high BDS and low hysteresis loss, however, a drastic reduction in the energy density was observed at elevated temperatures (Fig. 4(a)). Further, incorporation of Mn dopants into the CHT matrix significantly enhanced the BDS up to 1300 kV/cm and resulted in a much higher energy density of 9.6 J/cm3. Moreover, the temperature dependence of BDS and the ionic and electronic conductivities of the CHT ceramics were minimized (Fig. 4(b)).
In another study, Zhou et al.16) investigated the energy storage properties of Zr-doped CaTiO3 (CT) ceramics prepared by the conventional sintering method. With increasing concentration of Zr4+, the dielectric constant was found to decrease, while the BDS of the CT ceramics increased from 435 kV/cm to 756 kV/cm. As a result, the highest energy storage density of 2.7 J/cm3 was achieved for the CaZr0.4Ti0.6O3 system. The larger BDS achieved in the Zr-contained CT system is attributed to the decreased average field strength at the grain boundaries owing to its smaller grain size. Similarly, Mg-modified SrTiO3 (ST) ceramics exhibited finer grains with reduced grain activation energies, leading to much improved BDS (362 kV/cm) than pure ST ceramics (279 kV/cm); as a result, a larger Urec of 1.86 J/cm3 along with a higher efficiency (η) of 89.3% were achieved in the Sr0.99Mg0.01TiO3 system.18)
Since the BDS greatly depends on grain size, many researchers anticipated that grain size reduction is a feasible way to increase the DBS of a system. Zhao et al.19) produced fine-grained ST ceramics with an average grain size of 400 nm through the addition of SiO2, which led to an improvement in the BDS from 242 kV/cm to 361 kV/cm and resulted in a high Urec of 1.15 J/cm3. The addition of low temperature sintering aids such as ZnNb2O6 (ZN) and NiNb2O6 (NN) also decreased the grain size of ST-based ceramics, along with significant reductions in dielectric constant and dielectric loss. Moderate addition (6 wt.%) of ZN to Sr0.97Nd0.02TiO3 ceramics resulted in a high Urec of 2.37 J/cm3 at the applied electric field of 493 kV/cm.15) On the other hand, the addition of NN to Sr0.97La0.02TiO3 ceramics enhanced the BDS by boosting the grain boundary resistance, which led to an improved Urec of 1.36 J/cm3 (at 324 kV/cm).17)
Surface modifications of particles through high-resistance coatings were also employed to enhance the BDS of dielectric ceramics, where the coating acts as a shell and controls the grain growth occurring during sintering. Zeng et al.21) adopted the core-shell method to improve the BDS of ST ceramics by using SiO2 as a coating layer. The SiO2 layers with controlled thicknesses (2-13 nm) were coated on fine-grained ST particles by using Stöber process. The inter-diffusion occurring between the SiO2 shell and the ST cores during sintering facilitated grain growth suppression and secondary phase (Sr2TiSi2O8) formation, which resulted in enhanced BDS with reduced polarization. As the concentration of SiO2 increased, an enhancement in the BDS was observed up to 3 wt.% SiO2; above this concentration, BDS decreased due to abnormal grain growth. Optimized energy storage properties (Urec of 1.2 J/cm3 and η of 78.1% at 310 kV/cm) were realized for ST coated with 2.5 wt.% SiO2.
On the other hand, by taking advantage of doping with high permittivity (or high polarization) materials, it could be possible to enhance the energy storage properties of LD materials. In this fashion, Yang et al. obtained a high Urec of 2.59 J/cm3 along with an η of 85% even at the low electric field of 323 kV/cm in ST ceramics by doping with Bi0.48La0.02Na0.48Li0.02Ti0.98Zr0.02O3 (BLNLTZ).22) As shown in Fig. 4(c), the incorporation of BLNLTZ in ST ceramics significantly enhanced the saturation polarization from 7.95 μC/cm2 to 30.35 μC/cm2 by decreasing the BDS of the ST ceramics. In another study, substitution of BiScO3 into the crystal lattice of CaTiO3 resulted in improved dielectric and ferroelectric properties with enhanced BDS, which resulted in a higher Urec of 1.55 J/cm3 and η of 90.4% at 270 kV/cm, along with a power density of 1.79 MW/cm3 in 0.9CaTiO3-0.1BiScO3 ceramics.23) The enhanced energy storage properties were attributed to the enlarged bandgap caused by a strong hybridization between the O2p and Ti3d in the valence and conduction bands, as well as O2p and Sc3d hybridization in the conduction band.
Usually, enhancement in energy storage properties is achieved in LDs by compromising BDS or ɛr. It would be more beneficial if there is an improvement in both BDS and ɛr or an improvement in one property by maintaining the other property constant, though it is challenging. In this regard, few attempts have been made to improve the BDS as well as ɛr to realize high energy density properties. Zhou et al.20) produced fine-grained (~ 1 μm) high-density (99% of theoretical density) CaTiO3 (CT) ceramics by employing spark plasma sintering (SPS) technique. The SPS samples exhibited significantly improved dielectric properties (Fig. 4(d)) along with high BDS (Fig. 4(e)), resulting in a greatly enhanced Urec of 6.9 J/cm3 at the applied electric field of 910 kV/cm compared to that of conventionally sintered CT ceramics (Urec of 1.5 J/cm3 at 435 kV/cm). These remarkable properties of SPS-CT ceramics are attributed to the presence of discontinuous breakdown channels and microcrack networks, and the improved resistivity and thermal conductivity associated with small and uniform microstructures. In addition, with the introduction of amorphous alumina thin films in between the SPS-CT ceramic and the electrodes (SPS2), as shown in Fig. 4(f), a much improved BDS of 1188 kV/cm and Urec of 11.8 J/cm3 were obtained.
2.2. Paraelectric ceramics
In contrast to LD, nonlinear dielectrics such as PE ceramics having Curie temperatures below room temperature (RT) exhibit moderate ɛr and BDSs with low dielectric losses and weakly nonlinear P-E hysteresis that are promising for energy storage applications. Initially, Fletcher et al.24) demonstrated the feasibility of achieving the maximum energy storage density by using PE ceramic compositions (Sr-doped BaTiO3; BST) and realized energy storage of up to 8 J/cm3 at 1000 kV/cm. Afterwards, many efforts have been made to improve the energy storage performance of PE ceramics. Dong et al.25) investigated the energy storage properties of ZnO-doped Ba0.3Sr0.7TiO3 (BST + x wt.% ZnO (x = 0-5)) ceramics prepared by conventional sintering. The doping of ZnO to BST promoted densification and grain size reduction. As a result, improvements in the ɛr and BDS and a reduction in tanδ were observed at the optimal composition of BST + 1.6 wt.% ZnO, leading to a higher Urec of 3.9 J/cm3 at 400 kV/cm. In another study, enhancement in DBS and reduction in tanδ were observed at the expense of ɛr in BST ceramics doped with MgO nanopowder.26) The sample with the composition 0.7BST-0.3MgO exhibited the maximum Urec of 1.14 J/cm3 at 331 kV/cm.
Many studies have been conducted on the effect of grain size on the energy storage properties of BST-based ceramics as a result of the increasing requirement of miniaturization of electronic components.27-29) Song et al.27) prepared BST ceramics with different grain sizes (in the range 0.5-5.6 μm) by using the conventional sintering method and varying the sintering temperature. As the grain size decreased from 5.6 μm to 0.5 μm, ɛr decreased with the appearance of a diffuse-type transition (due to internal stress effect and polar nanoregions (PNRs)), whereas the BDS and maximum polarization were significantly enhanced. The improvement in the BDS is attributed to the enhanced grain boundary density. As a result, an improved Urec of 1.28 J/cm3 at 243 kV/cm was achieved in BST ceramics with an average grain size of 0.5 μm. In another study, BST ceramics of various grain sizes (0.405-1.635 μm) were synthesized by oxalate co-precipitation method and conventional and plasma-activated sintering (PAS) methods.28) Although the BST ceramics prepared through the PAS method exhibited small grains (0.405-0.550 μm) with a dense microstructure, the BDS (154-191 kV/cm) and Urec (0.63-0.94 J/cm3) values are very small owing to the smaller relative grain boundary resistance that resulted from residual oxygen vacancies (Rgb/(Rg+Rgb), where Rg and Rgb are the resistances of the grains and grain boundaries). On the other hand, the conventionally sintered samples displayed larger Rgb/(Rg+Rgb) values than the PAS samples, and showed an increasing trend with decreasing grain size, resulting in a larger Urec of 1.70 J/cm3 at 281 kV/cm that was obtained for the BST ceramic with grain size 0.66 μm.
Grain refinement had a great impact on the improvement of dielectric strength; however, it is difficult to achieve fine grains with uniform microstructures by using the conventional sintering route due to the uncontrolled grain growth that occurs during sintering. In this regard, various other sintering techniques such as microwave sintering (MWS)30) and SPS31,32) techniques have been employed to improve the energy storage properties of BST PE ceramics. Zhe et al.30) produced fine-grained (~ 0.65 ± 0.17 μm) BST ceramics with a dense and uniform microstructure by using the MWS method. The as-sintered MWS sample (at 990°C) exhibited high tanδ (> 0.1) and further thermal annealing in air at 1100°C for 10 h improved both the dielectric properties (ɛr ~ 910 and tanδ ~ 0.01) and the insulation properties. As a result, the thermally annealed MWS sample exhibited the maximum BDS of 180 kV/cm, compared to the conventionally sintered samples (130-154 kV/cm), which resulted in a larger Urec of 1.15 J/cm3 with an efficiency of 82%. In another study, Huang et al.31) investigated the SPS effect on the energy storage properties of BST ceramics. For this, ultrafine BST powders (45-105 nm) synthesized by sol-gel method and calcined at various temperatures (750-1050°C for 3 h) have been used. Further, the samples were sintered at 1000°C for 5 min. The rapid densification promoted by SPS allows direct grain boundary diffusion at higher temperatures by circumventing the surface diffusions that occur at low temperatures. As a result, highly dense microstructures (relative densities above 99%) with fine grains (173-238 nm) and few pores and cracks were obtained in the SPS sample. With increasing calcination temperature, enhancement in ɛr and BDS and reduction in tanδ were observed up to 950°C, which resulted in a maximum Urec of 1.23 J/cm3 at 240 kV/cm and efficiency of 94.5%; above this temperature, the properties slightly decreased. Usually, electrical breakdown in ceramics tends to occur at the weak links through the penetration of electrical currents at sufficiently high electric fields. However, electrical breakdown occurred in the SPS sample at relatively higher electric fields through the melting of grains rather than through the weak links. This significantly enhanced the BDS of the SPS sample containing fine grains with a pore-free microstructure, resulting in improved energy storage properties (Urec of 1.13 J/cm3 and η of 86.8%) compared to those of the conventionally sintered sample.32)
Since the sintering atmosphere also affects the microstructural and electrical properties of ceramic capacitors, some attempts have been made to investigate the effect of sintering atmosphere on the energy storage properties of BST-based paraelectric ceramics.33,34) Jin et al.33) synthesized BST powders by the hydrothermal method and subsequently sintered the ceramics in N2, air, and O2 atmospheres. The ceramics sintered in N2 and air atmospheres exhibited higher average grain sizes in the range 1.09-1.11 μm, whereas the sample sintered in O2 displayed an average grain size of 0.44 μm. The smaller grains with a homogenous microstructure in the O2-sintered sample could be attributed to the reduction in oxygen vacancies, which hindered grain growth as well as mass transport during sintering. Consequently, the O2-sintered sample displayed relaxor-like behavior with diffused-type transition (diffuseness coefficient = 1.618) and improved saturation polarization and BDS, which led to enhanced Urec of 1.08 J/cm3 and η of 73.8% at 167.5 kV/cm compared to the samples sintered in other atmospheres.
Gao et al.35) achieved high energy storage properties in Ba0.7Sr0.3TiO3-SrTiO3 (BST-ST) multilayer ceramics by taking advantage of the high polarization of BST ceramics and the high BDS of ST ceramics. A series of BST-ST multilayer ceramics have been fabricated by laminating various periodic combinations of the BST and ST layers. Introduction of the ST layer greatly decreased the dielectric loss and enhanced the BDS, however, a further increase in the number of ST layers drastically increased the leakage current due to the large accumulation of space charges and oxygen vacancies at the heterogeneous interfaces. At the optimal combination of B5S (i.e., a periodic combination of 5 BST layers and 1 ST layer), the multilayer ceramic exhibited a high BDS of 220 kV/cm and Urec of 2.3 J/cm3. Moreover, the BST-ST ceramics displayed good temperature- and frequency-stable dielectric properties.
In order to enhance the breakdown strength of the BST ceramics, the core-shell structure has been widely employed. Highly insulating oxides such as Al2O3 and SiO2 were used as coatings on PE particles.36-38) Huang et al. prepared core-shell structures of BST@SiO2 nanoparticles through the wet-chemical method and further sintered them by using the SPS technique.38) Upon increasing the SiO2 concentration of BST + x mol% SiO2 (i.e., x = 0, 5, 8, 12.5, 25, and 50), the average thickness of the SiO2 layer increased monotonously from 2.5 nm to 35 nm. The SPS sintered sample displayed a significant amount of the secondary phase (Ba,Sr)2TiSi2O8, and it’s intensity was enhanced with increasing SiO2 concentration. At lower concentrations (x ≤ 12.5) of SiO2, the BDS of the BST@SiO2 ceramics significantly improved up to 400 kV/cm at the expense of Pmax. However, excess amount of the SiO2 coating worsened both the BDS and Pmax of the BST@SiO2 ceramics. The BST ceramics containing 8 mol% SiO2 were the optimal combinations owing to their large Urec of 1.6 J/cm3 and η of 90.9%. In another study, BST nanoparticles coated with SiO2 and sintered using conventional sintering exhibited much improved energy storage properties such as a large Urec of 2 J/cm3 and an η of 80% even at the low electric field of 290 kV/cm.37) On the other hand, the BST ceramics fabricated from the nanoparticles coated with both Al2O3 and SiO2 layers could withstand a larger electric field of up to 493 kV/cm, as a result, an enormous energy storage density of 5.09 J/cm3 was achieved.36) Since glasses exhibit large BDS values, some attempts have been made to produce BST ceramics that can withstand higher electric fields by adding glasses. In this regard, Yang et al.39) studied the effect of glass (Bi2O3-B2O3-SiO2; BBS) addition on the energy storage properties of BST ceramics. The addition of glass to BST ceramics decreased the sintering temperature effectively and improved the frequency stability, BDS, and Pmax. The BST ceramics containing 9 wt% BBS exhibited excellent energy storage properties of Urec 1.98 J/cm3 and η 90.57% at 279 kV/cm.
With respect to the other family of BST ceramics, Zhang et al.40) attempted to study the energy storage properties of Ca-doped ST ceramics. The incorporation of Ca ions at the Sr-sites of the ST ceramics led to improvements in ɛr and BDS, while a reduction in tan δ was observed. At the applied electric field of 333 kV/cm, the Sr0.98Ca0.02TiO3 sample revealed a Urec of 1.95 J/cm3, with η being 72.3%, which is 2.8 times larger than that of pure ST ceramics.
Since the materials with a PE phase at RT display outstanding energy storage properties, many researchers explored the idea of shifting the Curie temperature (TC) of ferroelectrics through intentional doping. Zhou et al.41) succeeded in reducing the TC of the (Ba0.85Ca0.15)(Zr0.10Ti0.90)O3 (BCZT) ceramics to below RT by doping with a B-site (Ni1/3Nb2/3)4+ (NN) complex ion. The BCZT-NN ceramics showed a single-phase perovskite structure and good frequency and temperature stability. Furthermore, doping with NN reduced the P-E hysteresis loss and enhanced the BDS of BCZT ceramics. The maximum Urec of 0.66 J/cm3 with an η of 88.1% at 200 kV/cm was achieved in the case of BCZT-0.3NN. In another study, YNbO4 (YN) dopant was used to tailor the dielectric and ferroelectric properties of BT ceramics.42) With increasing YN concentration, the TC of (1-x)BT-xYN (x = 0-15%) ceramics was shifted from 130°C to below RT, along with a significant suppression in the dielectric nonlinearity and the P-E loops becoming slimmer with lower Pr and enhanced BDS values; as a result, a maximum Urec of 0.614 J/cm3 with an η of 86.8% at 173 kV/cm was obtained for 0.93BT-0.07YN, which was 2.4 times larger than that of the pure BT ceramic.
2.3. Ferroelectric ceramics
FE ceramics exhibiting nonlinear electric field dependent polarization characteristics with high saturation polarizations (high permittivities) are of particular interest in achieving superior energy storage properties under smaller electric fields. However, the high dielectric loss and fat P-E hysteresis loops resulting from domain switching enforce the low BDS as well as smaller energy storage density of FE ceramics. Among the various FE ceramics, a few efforts have been made to improve the energy storage properties of (Bi, Na)TiO3 (BNT),43-45) Ba(Zr, Ti)O3 (BZT),46-48) BaTiO3 (BT),49-52) and (K, Na)NbO3 (KNN)53) based ceramics. Gao et al.43) studied the effect of tetragonality ratio (c/a ratio) on the energy storage properties of (0.9-x) Bi0.5Na0.5TiO3-xBa-TiO3-0.1K0.5Na0.5NbO3 (x = 0.060-0.069) [BNT-BT-KNN] ceramics by varying the BNT/BT ratio. As the BNT/BT ratio increased, the c/a ratio also increased initially (up to x = 0.063), and then decreased. The sample having a larger c/a ratio (~ 0.709) exhibited the maximum value of Ps-Pr, which led to an enhanced Urec of 0.424 J/cm3 at 50 kV/cm. The c/a ratio dependence of the Urec of BNT-BT-KNN ceramics is depicted in Fig. 5(a), which reveals an almost linear relation. In another study, highly dense BCT and BZT ceramics prepared by conventional sintering exhibited larger c/a ratios of 1.027 and 1.002, as a result, much improved energy densities of 1.41 J/cm3 (η of 61%) and 0.71 J/cm3 (η of 19%) at 150 kV/cm, respectively, were realized.47) On the contrary, in spite of the decrease in the c/a ratio from 1.010 to 1.003, NaNbO3 (NN) modified 0.92BaTiO3-0.08K0.5Bi0.5TiO3 (BTKBT) ceramics displayed much improved energy storage properties (Urec of 1.96 J/cm3 and η of 67.4% at the electric field of 220 kV/cm) due to the reduction in grain size that was facilitated by the defect dipoles (
V N a ′ - V O ¨ - V N a ′
Sreenivas et al.46) investigated the energy storage properties of (1-x) BZT-x BCT (x = 0.10-0.30) based ceramic capacitors sintered at 1600°C. For all compositions, the ceramics exhibited a single-phase perovskite structure without any secondary phases and the average grain sizes were in the range 20-30 μm. The ceramic corresponding to x = 0.15 displayed a relatively higher ɛr (8400) with low tan δ and large BDS of 170 kV/cm, leading to an enhanced Urec of 0.68 J/cm3 with an η of 72%.
Microstructural modification of FE nanoparticles was also adopted for improving the energy storage properties of FE ceramics. Ma et al.50) utilized the double coating technique to prepare multilevel core-shell structures of BT@La2O3@SiO2 particles. The combined effects of structural distortion, which led to an improvement in the dielectric property due to La2O3 and the acceleration of sintering, as well as density promotion by SiO2, led to enhanced energy storage properties (Urec of 0.54 J/cm3 and η of 85.7% at 136 kV/cm) in BT@La2O3@SiO2 ceramics containing 9 wt% SiO2. However, excess amounts of SiO2 (> 9 wt%) promoted the interface reaction and the formation of the Ba2TiSi2O8 secondary phase, which lead to the collapse of the energy storage properties.
It is well known that fine grains with pore-free structures are favorable for improving the BDS and energy density properties of ceramics. Since the microstructural features of ceramics are affected by the fabrication method, many researchers have attempted to tailor the energy storage properties of the FE ceramics fabricated by various methods.45,51) Xu et al.45) investigated the energy storage properties of (1-x) 0.93BNT-0.07BT-x KNbO3 (x = 0-0.07) (BNTBT-KN) ceramics synthesized via wet-chemical method and sintered using the conventional method. Initially, the BNT-BT and KN powders were synthesized by sol-gel and hydrothermal methods to obtain fine powders and the stoichiometric mixtures were sintered at 1050-1175°C for 2 h. The ceramic with x = 0.05 (5KN) exhibited the maximum Urec of 1.72 J/cm3 owing to its higher BDS (168 kV/cm), increased ɛr (1550), and reduced grain size (1.03 μm) compared to those of pure BNTBT ceramics. Moreover, the wet-chemical synthesized 5KN ceramics displayed an almost 90% enhanced BDS and 74% enhanced recoverable energy density compared with the 5KN ceramics prepared via the solid-state method (Urec of 0.99 J/cm3 at 88.5 kV/cm). In another study, Ma et al.51) demonstrated the feasibility of achieving fine-grained highly dense BT ceramics even at sufficiently low temperatures by employing the cold sintering method. The pellets were prepared from hydrothermal precursors of BT nanoparticles and cold sintered at 180°C for different dwelling times (15-120 min). Subsequently, all sintered samples were dried and annealed in air at 900°C for 3 h to induce densification. Among all the samples, that sintered for 60 min exhibited a relatively high density (96.8%) and improved dielectric properties (ɛr of 2332 and tan δ of 0.01 at 1 kHz). The fine grains associated with the high density resulted in an improved energy density of 1.45 J/cm3 with an η of 85.6% at the applied electric field of 90 kV/cm.
By utilizing the potential of sintering aids to reduce the sintering temperature, Qu et al.53) produced highly dense fine-grained 0.9(K0.5Na0.5)NbO3-0.1Bi(Mg2/3Nb1/3)O3 (KNN-BMN) ceramics through liquid phase sintering. The sintering temperatures of the KNN-BMN ceramics were effectively decreased from 1150°C to 930°C with the addition of CuO, as shown in Fig. 5(b). Moreover, CuO-modified KNN-BMN (KNN-BMN-1.0 mol% CuO) ceramics could withstand high electric fields of up to 400 kV/cm owing to their dense microstructure without any porosity and displayed a large polarization difference (Ps-Pr) due to the strong hybridization between Bi6p and O2p, leading to exceptional energy storage properties (Urec of 4.02 J/cm3 and η of 57.3%) (Fig. 5(c-d)).
2.4. Relaxor ferroelectric ceramics
RFE ceramics are drawing much attention for energy storage applications owing to their outstanding dielectric and FE properties. Similar to the FE, RFE materials also exhibit large permittivities and saturation polarizations, but possess lower remnant polarizations and slimmer hysteresis loops, which are essential for realizing extremely high energy densities and efficiencies. These materials are mainly characterized by the broad frequency-dependent peak of the temperature-dependent dielectric susceptibility and the slim P-E hysteresis loops. The RFE behavior is assumed to originate from the PNR, which usually appears below Burn’s temperature.
2.4.1. Lead-based RFE ceramics
Quite a few lead-based relaxor ceramics such as PbZrO3-(SrTiO3),54) Pb(Mn1/3Nb2/3)O3-PbTiO3,55-57) and La-doped Pb(Zr, Ti)O358-60) were examined for energy storage applications by considering the human health and environmental concerns and their thermal stabilities. Recently, Zhang et al.54) investigated the energy storage performance of (1-x) PbZrO3-x SrTiO3 (PZ-ST) (x = 10-30 mol%) ceramics prepared by the solid state reaction method. The incorporation of ST in the PZ matrix induced relaxor behavior by transforming the macrodomains of PZ into microdomains, which resulted in slim hysteresis loops with enhanced saturation polarizations. As a result, a maximum Urec of 0.46 J/cm3 with an η of 79.3% at the applied electric field of 79.3 kV/cm was achieved in 0.7PZ-0.3ST relaxor ceramics. Moreover, 0.7PZ-0.3ST ceramics displayed oxygen-vacancy (Vö)-induced high-temperature dielectric relaxation. In another study, Zhang et al.57) reported relaxor behavior in Pb(Mn1/3Nb2/3)O3-PbTiO3 (PMN-PT) ceramics that was induced by the formation of complex defect dipoles Vö-Nb4+. Li et al.58) investigated the effect of excess amount of lead (PbO) on the energy storage properties of (Pb0.97(1+x)La0.02)(Zr0.95Ti0.05)O3 (PLZT2/95/5) (x = 0-15%) ceramics. As the concentration of PbO increased, the Urec of the PLZT2/95/5 ceramics increased from 0.36 J/cm3 to 1.94 J/cm3 at the electric field of 90 kV/cm due to the increased dielectric relaxation resulting from the volatilization of PbO during sintering. Further, Urec also showed temperature dependence and reached a maximum value of 2.12 J/cm3 (η of 92.98%) at 120°C due to the AFE to RFE phase transition in the PLZT2/95/5 ceramics containing more than 10% PbO. Besides, Gao et al.59) reported that the (Pb0.9La0.1)(Zr0.65Ti0.35)O3 (PLZT10/65/35) ceramics displayed good temperature stability with a slight variation in Urec (< 15%) over the temperature range 24-83°C, when measured at 25 kV/cm. This temperature stability results from the continuous formation and growth of PNRs with temperature, as well as the applied electric field. In addition, the other PLZT ceramics ((Pb0.88La0.08)(Zr0.91Ti0.09)O3; PLZT8/91/9) also exhibited good fatigue lives of up to 105 electric field cycles without any significant degradation.60) Moreover, the PLZT8/91/9 ceramics were able to withstand large electric fields of up to 170 kV/cm, leading to the realization of a superior Urec of 3.04 J/cm3 and an η of 92%.
2.4.2. Lead-free RFE ceramics
(a) BaTiO3-based RFE ceramics
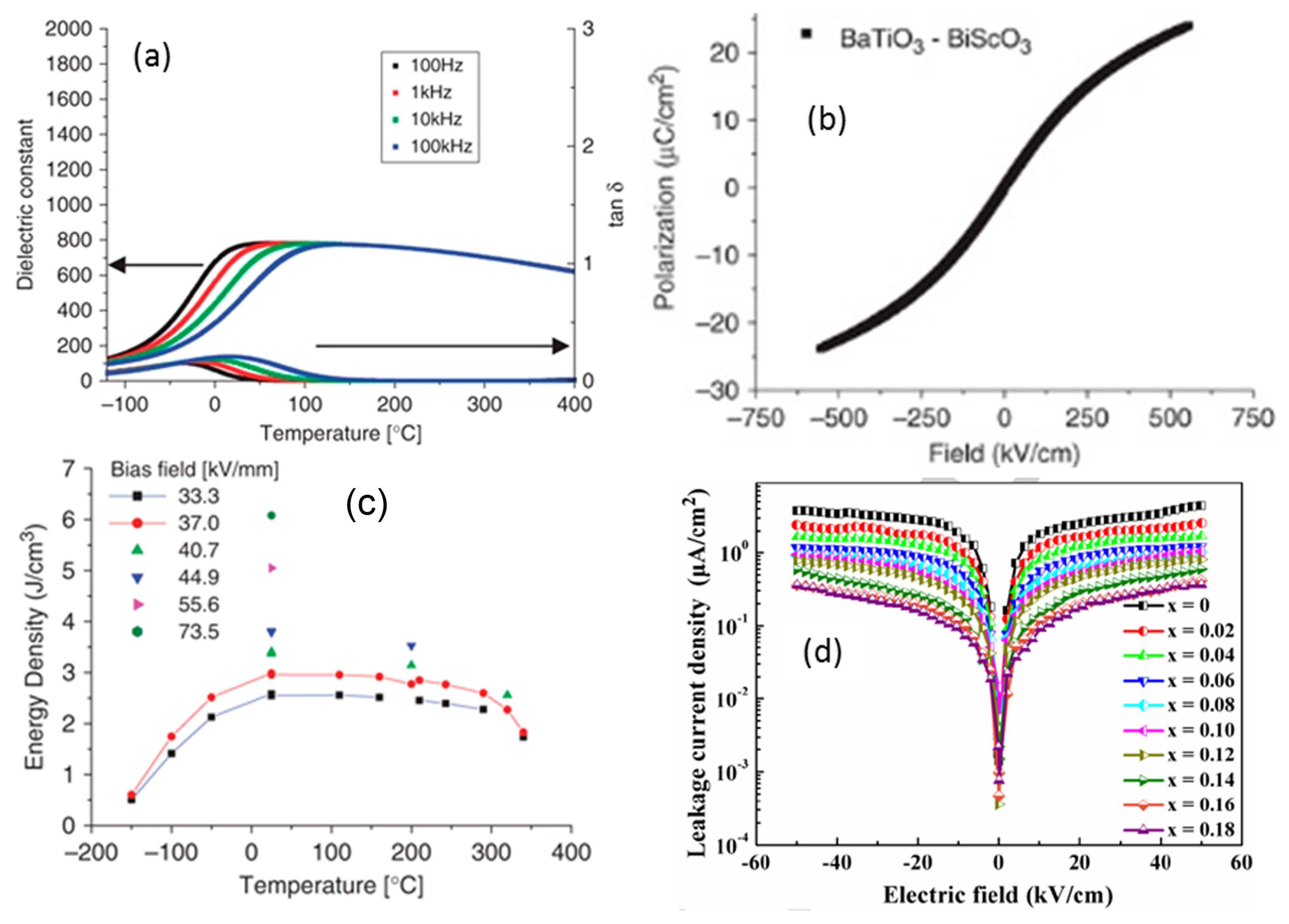
BaTiO3-based RFE ceramics and solid solutions are considered as one of the most promising candidates for energy storage applications. Ogihara et al.61) demonstrated the high-temperature stable energy storage properties of 0.7Ba-TiO3-0.3BiScO3 (BTBS) ceramics prepared via the tape casting method. From Fig. 6(a), the appearance of diffusive and dispersion phase transitions is indicative of the relaxor behavior of BTBS ceramics, which is also evidenced from the slim P-E loops (Fig. 6(b)). Further, the BDS was found to increase with decreasing thickness of the capacitor, and relatively high BDS (730 kV/cm) and Urec (6.1 J/cm3) were obtained for a 15 μm thick BTBS single layer capacitor. Moreover, the BTBS capacitors exhibited good thermal stability over a wide temperature range 0-300°C (Fig. 6(c)), and the obtained results are superior to those of the commercially available capacitors. Since then, many researchers have investigated the energy storage performance of various BT-based RFE solid solutions such as BaTiO3-Bi(Mg2/3Nb1/3)O3,62,63) BaTiO3-Bi(Mg1/2Nb1/2)O3,64,65) BaTiO3-Bi(Zn2/3Nb1/3)O3,66) and BaTiO3-Bi0.5Na0.5TiO3-Na0.73Bi0.09NbO3.67) Wang et al.62) reported that the addition of Bi(Mg2/3Nb1/3)O3 (BMN) to BT ceramics induced relaxor behavior that was attributed to the large difference in ionic radii of B-site cations and A-site cations. With increasing concentration of BMN, the diffused phase transition became flat and exhibited good temperature stability (ɛr ~ 628-787) over a wide temperature range (−50-300°C). Moreover, the decrease in ɛr and increase in resistivity with BMN concentration leads to an improvement in the BDS of BT-BMN ceramics, which helps to enhance the Urec. The highest Urec of 1.13 J/cm3 with an η of 96% at the applied electric field of 143.5 kV/cm was achieved for 0.9BT-0.1BMN. Li et al.63) prepared MnCO3-doped 0.9BT-0.1BMN ceramics to improve the energy storage properties by mitigating the dielectric as well as ferroelectric losses. As reported, acceptor (Mn2+) doping-induced dipoles (
Mn 2 + - V o ″
(b) (Na, Bi)TiO3-based RFE ceramics
Among the lead-free ceramics, (Na, Bi)TiO3 (NBT)-based FE ceramics were extensively studied owing to their excellent ferroelectric properties that can be ascribed to the (Na, Bi)2+ ions, especially the lone-pair 6s2 electronic configuration of Bi3+.71) At RT, NBT exhibits a rhombohedral FE structure that transforms to a weakly polar tetragonal phase at elevated temperatures, which is either a RFE or an AFE phase. Therefore, many efforts have been made to induce relaxor behavior and improve the BDS of the NBT ceramics by doping and forming binary/ternary solutions.
Recently, Liu et al.72) reported the energy storage properties of BNT-based binary solutions such as Ba0.06Na0.47Bi0.47TiO3-Ln1/3NbO3 (BNBT-LnN, Ln = La, Nd, Sm) ceramics. The introduction of LnN in BNBT ceramics significantly reduced the polarizations (Pr and Pmax) and promoted the relaxor behavior, which lead to a maximum Urec of 1.239 J/cm3 at 100 kV/cm in the case of 0.98BBNT-0.02SmN. The deliberate doping of Sr0.85Bi0.1TiO3 (SBT) in NBT ceramics induced ergodic relaxor behavior with reduced Pr and improved Pmax, as a result, a larger Urec of 1.5 J/cm3 and an η of 73% were achieved under a relatively lower electric field (85 kV/cm).73) In addition, the BDS and ionic conductivity of the BBNT ceramics were enhanced, whereas the Pr, Pmax, and electronic conductivity reduced with SrZrO3 (SZ) doping.74)
Pu et al.75) investigated the effect of Sn4+ doping on the energy storage properties of 0.55Bi0.5Na0.5TiO3-0.45Ba0.85-Ca0.15Ti0.9Zr0.1O3 (0.55BNT-0.45BCTZ) ceramics. With the increase in Sn4+ concentration, a diffuse-type phase transition with broad plateau-like dielectric constant maxima over a wide temperature range was observed. In addition, the low ionic polarizability of Sn4+ facilitated the softening of the current peaks and the reduction of hysteresis loss. As a consequence, improved energy storage properties (Urec of 1.21 J/cm3 and η of 72.29% at 130 kV/cm) were realized in 0.55Bi0.5Na0.5TiO3-0.45Ba0.85Ca0.15Ti0.85Zr0.1Sn0.05O3 (0.55BNT-0.45BCTZS) ceramics. In an extension of this study, the energy storage properties of BNT-BCTZ were further enhanced by doping with MgO and utilizing microwave sintering.76,77)
Since reduction in leakage current is an effective way to enhance the BDS of ceramics, Yang et al.70) considered La3+ doping for minimizing the remnant polarization and leakage current of BNT-based ceramics. With increasing La3+ concentration in Bi(0.5-x)Lax(Na0.82K0.18)0.5Ti0.96Zr0.02Sn0.02O3 (BNKTZS-xL) (x = 0-0.18) ceramics, a gradual decrease in grain size, as well as structural transition with enhanced relaxor behavior, was noticed. Moreover, BNKTZS-xL (x = 0.06-0.18) samples exhibited good temperature stability with improved temperature coefficient of capacitance (≤ ± 15% at 150°C). The polarization (Pr and Pmax) properties and leakage current density (Fig. 6(d)) of BNKTZS-xL ceramics decreased continuously with increasing x, as a result, the hysteresis loss decreased, while the BDS was enhanced from 80 kV/cm to 155 kV/cm. A maximum Urec of 1.95 J/cm3 with an η of 71% was obtained for BNKTZS-0.1L. In another study, the introduction of BaSnO3 (BSN) into NBT ceramics also suppressed the phase transition temperature as well as the leakage current peaks and increase the ability of the ceramics to withstand higher electric fields, which in turn resulted in the BDS increasing from 140 kV/cm to 240 kV/cm and yielded a maximum Urec of 1.91 J/cm3 and η of 86.4% for 0.75NBT-0.25BSN.78)
On the other hand, BNT-based ternary systems based on the solid solutions of various ABO3-type ferro/non-ferroelectrics at the morphotrophic phase boundary (MPB) were also extensively studied to expand the thermal stability range as well as to increase the energy storage capacity by shifting the transition temperature (especially Tm) towards RT.79) Among the BNT-based ternary systems, (Bi0.5Na0.5)TiO3-(Bi0.5K0.5)TiO3-(K0.5Na0.5)NbO3,80) Bi0.5Na0.5TiO3-BaTiO3-Na0.73Bi0.09NbO3,81) BiTi0.5Zn0.5O3-Bi0.5Na0.5TiO3-BaTiO3,82) Bi0.5Na0.5TiO3-NaNbO3-Ba(Zr0.2Ti0.8)O3,83) Bi0.5Na0.5TiO3-BaTiO3-NaTaO3,84) Na0.5Bi0.5TiO3-BaTiO3-NaNbO3,85) and Bi0.48La0.02Na0.48Li0.02Ti0.98Zr0.02O3-Na0.73Bi0.09NbO386) exhibited excellent energy storage properties (> 1 J/cm3) with good temperature stability and fatigue-free behavior.
(c) (K0.5Na0.5)NbO3 (KNN)-based RFE ceramics
A Few studies have been dedicated to improving the DBS of KNN-based ceramics through compositionally controlled grain size and porosity reduction.87-91) Qu et al. achieved a high energy storage density in Sr(Sc0.5Nb0.5)O3 (SSN)-doped fine-grained KNN ceramics ((1-x)KNN-xSSN, x = 0-0.3) prepared by conventional sintering.87) The crystal structure of the KNN ceramics gradually transformed from orthorhombic symmetry to cubic symmetry, accompanied by a decrease in grain size from 4 μm to 0.4 μm, and a strong frequency dispersion of ɛr with diffuse phase transitions were observed with increasing concentration of SSN. Moreover, the small grain size together with reduced porosity favored good transparency in the visible region as well as enhanced DBS up to 295 kV/cm, as a result, a larger Urec of 2.02 J/cm3 with an η of 81.4% were realized in 0.8KNN-0.2SSN ceramics. The addition of a sintering aid such as ZnO further enhanced the DBS from 295 kV/cm to 400 kV/cm, resulting in an improved Urec of 2.6 J/cm3 and an η of 73.2% for 0.8KNN-0.2SSN ceramics containing 0.5 mol% ZnO.89) Inspired by these results, different dopants such as ST,88) Sr(Zn1/3Nb2/3)O3 (SZN),90) Bi2O3,92) and Bi(Mg2/3Nb1/3)O3 (BMN)91) were used to reduce the average grain size and improve the BDS of KNN ceramics and obtain the maximum Urec (η) values of 4.03 J/cm3 (52%) at 400 kV/cm, 1.5 J/cm3 (50%) at 175 kV/cm, 1.04 J/cm3 at 189 kV/cm, and 4.08 J/cm3 (62.7%) at 300 kV/cm.
(d) Other lead-free RFE ceramics
This section provides an overview of the energy storage performances of various solid solutions of BiFeO393-95) and ST96-103) based lead-free RFE ceramics. A few attempts have been made to study the doping effect of low dielectric loss materials such as Ba(Mg1/3Nb2/3)O3 (BMN)93) and La(Mg1/2Ti1/2)O3 (LMT)94) on the energy storage performance of 0.67BiFeO3-0.33BaTiO3 (0.67BF-0.33BT) ceramics. The doping of these materials favored the decrease in the size of PNRs and enhanced their dynamics, consequently, a steady decrease in Pr and shifting of temperature (Tm) corresponding to the ɛr maxima towards RT with the increase in dielectric relaxation behavior (i.e., increased values of γ and ΔTrelax = Tm@1kHz - Tm@1MHz with increasing dopant concentration) and temperature stability were observed. Moreover, the coexistence of ergodic and non-ergodic behaviors at RT in optimal compositions lead to improved energy storage properties (Urec = 1.56-1.66 J/cm3 and η = 75-82%) even under small electric fields (125-130 kV/cm). In another study, the doping of the less polarizable Nd in Bi-sites stabilized the pseudocubic phase of BF-BT ceramics, with decreases in Tm and ferroelectricity.95) At the optimal composition of 0.75(Bi0.85Nd0.15)FeO3-0.25BaTiO3 + 0.1 wt.% MnO2 (BN15F-BT), a maximum Urec of 1.82 J/cm3 with an η of 41.3% at 170 kV/cm was achieved. By utilizing the advantage of interfacial modification in improving the BDS of BN15F-BT ceramics, the authors fabricated a multilayer capacitor (of thickness 0.78 mm) by using an optimized composition of BN15F-BT and Pt internal electrodes, which helped the ceramics withstand electric fields of up to 540 kV/cm and yielded a much improved Urec of 6.74 J/cm3 and η of 77%. Moreover, the multilayered BN15F-BT capacitor exhibited fast discharging (τ0.9 ~ 4 μs, the time required to release 90% of its total stored energy) and good thermal stability (ΔUrec ~ 15%) in the temperature range 30-125°C.95)
It is well known that ST possesses high BDS and η, but low Pmax. In order to achieve a high Urec along with high η, many researchers adopted compositional modification to improve the Pmax (or ɛr) of ST-based ceramics.96-103) For example, the incorporation of (Na0.5Bi0.5)2+ and Ba2+ in the A-sites of ST ceramics increased the lattice disorder as well as the growth of PNRs, which in turn enhanced the relaxor behavior along with improved polarization properties.98,99,102,103) Further, the addition of ZrO2 improved the BDS by stabilizing the pseudocubic phase and charge on Ti and suppressing the dissociation of oxygen during the sintering of 0.6ST-0.4NBT ceramics.100) As the ZrO2 concentration increased from 0.1 mol% to 0.5 mol%, the BDS increased from 220 kV/cm to 285 kV/cm, as a result, a maximum Urec of 2.84 J/cm3 with an η of 71.54% has been achieved. Similarly, Sn4+ doping also effectively reduced the average grain size and dielectric loss and improved the BDS as well as the energy storage properties of 0.45ST-0.2NBT-0.35BT ceramics.103)
Yan et al.96) reported that doping of 0.95Bi0.5Na0.5TiO3-0.05BaAl0.5Nb0.5O3 (NBT-BAN) significantly enhanced the Pmax (from 4.70 μC/cm2 to 41.81 μC/cm2), with a slight increase in Pr, which was accompanied by a 10.39 times improvement in the dielectric constant of the ST ceramics; this led to the realization of a high Urec of 1.89 J/cm3 with η being 77% at 190 kV/cm for the 0.5ST-0.5(NBT-BAN) system. Yang et al.101) reported an improvement in the energy storage properties (Urec of 2.83 J/cm3 and η of 85% at 320 kV/cm) of 0.93Bi0.5Na0.5TiO3-0.07Ba0.94La0.04Zr0.02Ti0.98O3 (NBT-BLZT)-doped ST ceramics due to the improvement in Pr-Pmax and grain size.
2.5. Antiferroelectric ceramics
The absence of FE dipoles at smaller electric fields and the field-induced reversible FE phase at higher fields lead to low remnant and high saturation polarizations in AFE materials, which make them promising for energy storage applications.13,104) Among the various AFE materials, PbZrO3 (PZ), (Na, Bi)TiO3 (NBT), and AgNbO3 (AN) based ceramics have been widely investigated for their suitability for energy storage applications. Since a large switching electric field (EAFE-FE) and slim hysteresis (i.e., small ΔE = EAFE-FE-EFE-AFE) along with large polarization are required to obtain high energy storage densities in AFE ceramics, various approaches such as chemical modification, different fabrication methods, and mechanical confinement have been adopted.
2.5.1. Lead-based AFE ceramics
Sawaguchi et al. demonstrated that AFE behavior in PZ originated from the antiparallel displacement of Pb2+ in the plane perpendicular to the c-axis.105) For the stabilization of the AFE phase in PZ ceramics, various dopants such as Ba, La, Sr, Sm, or Y in the Pb-sites and Nb, Sn, or Ti in the Zr-sites have been used. Among the various lead-based materials, (Pb, La)(Zr, Ti)O3 (PLZT),106,107) (Pb, La)(Zr, Sn, Ti)O3 (PLZST),108-120) Pb(Nb, Zr, Sn, Ti)O3 (PNZST),121,122) (Pb, La) (Nb, Zr, Sn, Ti)O3 (PLNZST),123) (Pb, La, Ba)(Zr, Sn, Ti)O3 (PLBZST),124-130) (Pb, La, Ba, Y)(Zr, Sn, Ti)O3 (PLBYZST),131-134) (Pb, Sm)(Zr, Sn, Ti)O3 (PSZST),135) and Pb(Tm, Nb)O3-Pb(Mg, Nb)O3 (PTN-PMN)136) based AFE ceramics have been considered as promising candidates for energy harvesting applications.
After the establishment of the triaxial phase diagram of the La3+-doped Pb(Zr, Sn, Ti)O3 system,137) the PLZST-based AFE system has been extensively investigated for energy storage applications.108-120) At RT, the PLZST AFE ceramics exist in either the tetragonal (AFET) phase or the orthorhombic (AFEO) phase, depending on the composition; the ceramics with the AFEO phase display relatively higher EAFE-FE than those with the AFET phase. In this regard, some efforts have been made to improve the stability of the AFEO phase of the PLZST ceramics via compositional modification.112,114,116,118,120) For example, Wang et al.112) tailored the Zr/Sn and Zr/Ti ratios of PLZST ceramics and realized improved energy storage properties for Zr-rich compositions due to the increase in AFEO phase stability. A higher EAFE-FE (227 kV/cm) along with a square-type hysteresis loop (ΔE ~ 90 kV/cm) and maximum polarization (30 μC/cm2) were achieved for Zr/Sn = 90/05. Although larger EAFE-FE (275 kV/cm) and slanted hysteresis (ΔE ~ 28 kV/cm) loops were realized in Zr/Ti = 92/03 composition, Pmax was quite low. The composition of Pb0.97La0.02(Zr0.90Sn0.05Ti0.05)O3 was optimal owing to its high Urec of 4.426 J/cm3 at 300 kV/cm with an η of 61.2%. In another study, Zhang et al.118) reported that the substitution of Zr4+ for Sn4+ transformed the crystal structure of PLZST ceramics from AFET to AFEO and shifted the AFE-FE and FE-AFE transitions towards higher electric fields, which led to an improvement in Urec from 3.18 J/cm3 to 4.38 J/cm3. In a recent study, Liu et al.120) studied the effect of different concentrations of Zr and Ti on the phase transition behavior and energy storage performance of tetragonal structured Pb0.97La0.02(ZrxSn0.925-xTi0.075)O3 (x = 0.58-0.82) and Pb0.97La0.02(Zr0.58Sn0.42-yTiy)O3 (y = 0.07-0.11) systems. Both systems exhibited small EAFE-FE and EFE-AFE, with large DE values at the MPB compositions. As the Zr concentration increased (at a fixed Ti concentration), both EAFE-FE and EFE-AFE linearly decreased, while DE increased; as a result, Urec as well as η were reduced. On the other hand, decreasing the Ti concentration (at a fixed Zr concentration) resulted in improved energy storage properties (Urec ~ 2.35 J/cm3 and η ~ 86% for y = 0.07) due to increases in both EAFE-FE and EFE-AFE. A few attempts have been made to replace Pb2+ (1.49 Å) with smaller ionic radii elements (Sr and Sm) to reduce the tolerance factor, which can help to improve the stability of the AFE phase of PLZST ceramics.116,117,135) Zhang et al.116) reported that with the incorporation of the inert-gas-type outermost electron configuration (4s24p6) of Sr2+ in Pb2+ (6s2, non-inert type), the crystal structure of (Pb0.97-xSrxLa0.02)(Zr0.75Sn0.195Ti0.055)O3 (x = 0-0.025) changed from AFET to AFEO, ɛrm and Pmax decreased, and the Tm and switching fields (EAFE-FE and EFE-AFE) increased. As a result, the Urec increased with increasing Sr2+ concentration and reached a maximum of 5.56 J/cm3, with the η being 67.9% at 350 kV/cm for x = 0.015. In addition, the PLZST-based ceramics exhibited good temperature stability and released their stored energy in less than 200 ns.115-117)
A few studies have attempted to investigate the effect of Ba doping on the energy storage properties of PLZST ceramics.124,128) The doping of Ba2+ in Pb2+ sites led to enhanced energy storage properties due to the improvements in ɛr and Pmax, however, significant decreases in the switching fields and phase transition temperatures were noticed. On the other hand, the incorporation of the smaller ion Y3+ to substitute Pb2+ lead to decreases in oxygen vacancies and the grain size of (Pb0.87Ba0.1La0.02) (Zr0.65Sn0.3Ti0.05)O3 + x mol% Y (PBLZST-xY, x = 0-1.25) ceramics, which facilitated an increase in the internal stress between the grains; therefore, higher switching fields and smaller ΔE were observed.131) For low concentrations (up to 0.75 mol%) of Y, the Urec of the PBLZST ceramics increased from 2.20 J/cm3 to 2.75 J/cm3, however, at higher concentrations, it decreased due to the reduction in Pmax. Since the electrical properties of Pb-based AFE ceramics are mainly affected by the density and lead loss during sintering, Zhang et al.127) adopted the hot-press (HP) method and added excess PbO to improve the electrical properties of PBLZST + 0.75 mol% Y ceramics. The HP samples exhibited improved microstructures, with relative densities of more than 98%, and enhanced resistivity compared to the samples obtained by the conventional sintering method. Moreover, hot-pressed PBLZST + 0.75 mol% Y with an appropriately excess amount of PbO (6 mol%) displayed a much higher BDS and Pmax, leading to a high Urec of 3.2 J/cm3 (at 180 kV/cm), which is almost twice that of the CS sample (1.6 J/cm3).
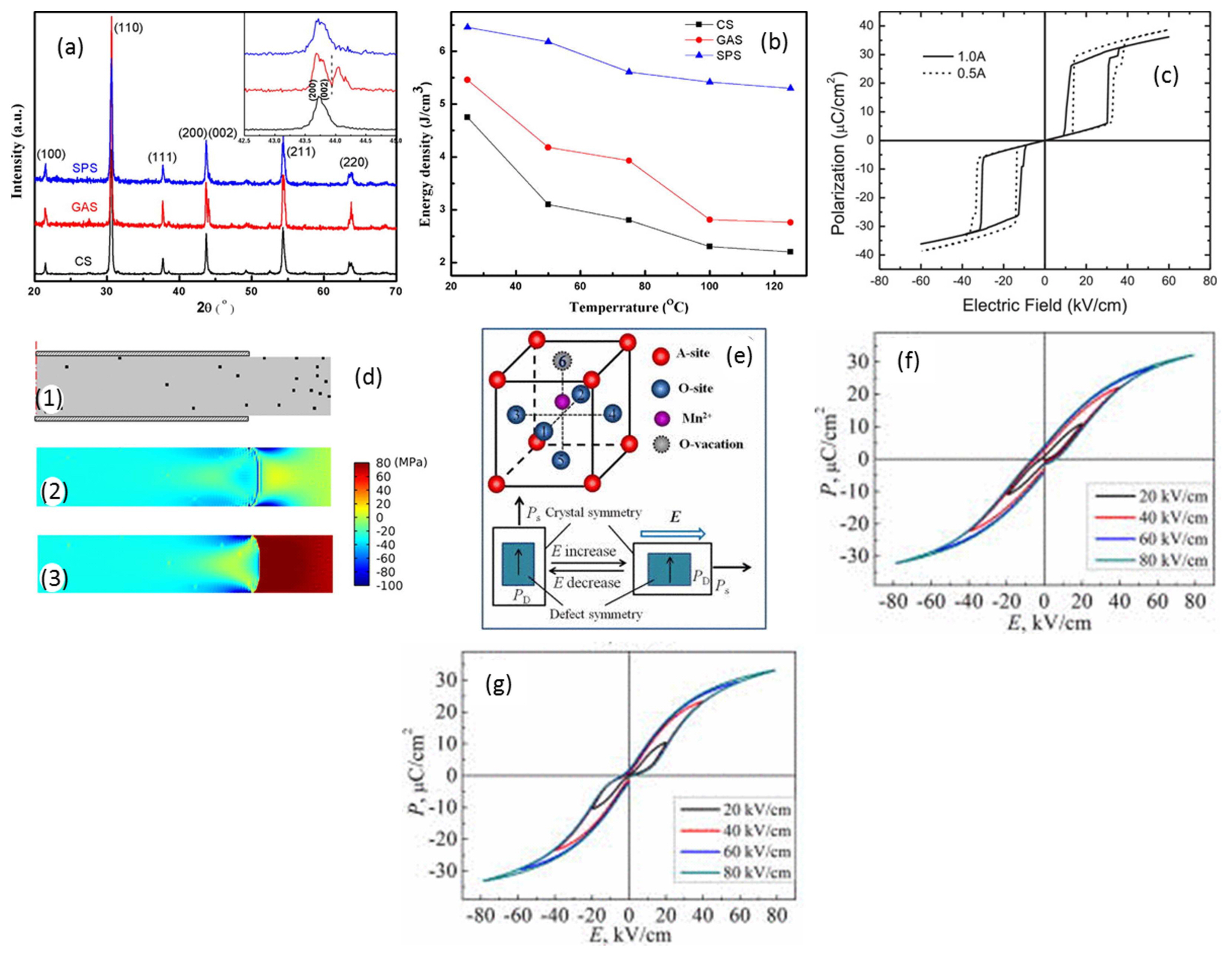
The AFET ceramics can possess larger Pmax but smaller switching fields compared to those of the AFEO ceramics; by utilizing the large switching field (or large BDS) of the AFEO ceramics, it could be possible to obtain improved Urec in AFET and AFEO two-phase composite ceramics. In this context, Zhang et al.132) investigated the energy storage properties of (Pb0.858Ba0.1La0.02Y0.008)(Zr0.65Sn0.3Ti0.05)O3-x(Pb0.97La0.02) (Zr0.9Sn0.05Ti0.05)O3 (PLBYZST-PLZST, x = 0-100 wt%) AFE composite ceramics. As the PLZST concentration was increased from 0 to 50 wt%, EAFE-FE increased from 80 kV/cm to 130 kV/cm without a significant decrease in Pmax, resulting in an improved Urec of 4.65 J/cm3 with an η of 60% at 200 kV/cm. Moreover, the dielectric temperature stability was enhanced through the shifting of both the dielectric peaks (AFEO-AFET and AFET-PE) towards higher temperatures. However, both EAFE-FE and Pmax decreased when the PLZST concentration exceeded 50 wt%, due to enhanced compositional heterogeneity and the appearance of a secondary phase. In order to suppress the diffusion occurring between the two phases of PLBYZST-PLZST AFE composite ceramics during sintering, the SPS method and glass-aided sintering (GAS) have been employed.133,134) As can be seen from Fig. 7(a), there is a clear split in the (002) and (200) peaks for the SPS and GAS samples compared with the case of the conventionally sintered samples, indicating reduced diffusion, which helped in improving the contributions of both the AFEO and AFET phases.134) As a result, much higher Urec were realized for the SPS (6.46 J/cm3) and GAS (5.46 J/cm3) samples compared to the conventionally sintered sample (4.65 J/cm3) that can be attributed to the increased AFE-FE transition and improved saturation polarization (Fig. 7(b)). In addition, the SPS sample displayed good temperature stability (25-125°C) relative to the other samples owing to its higher AFEO-AFET transition temperature.
It is well known that Pb-based complex perovskites such as Pb(B1/2Nb1/2)O3 (B = Lu, In, Yb, Tm) exhibit typical AFE characteristics and good temperature stability, though not many studies have been dedicated to evaluating their energy storage properties owing to their low saturation polarization. Recently, Xu et al.136) used Pb(Mg1/3Nb2/3)O3 (PMN) as a dopant to stabilize the perovskite phase and modulate the polarization and switching fields of Pb(Tm1/2Nb1/2)O3 (PTmN) ceramics. All the (1-x)PTmN-xPMN (x = 0.02-0.18) specimens exhibited the pure perovskite structure with an orthorhombic phase. Continuous decreases in the unit cell volume, structural ordering of the B-site cations, TC, and EAFE-FE and progressive improvements in Pmax and ɛr with increasing concentration of PMN were observed. This continuous increase in Pmax and the decrease in EAFE-FE led to the maximum Urec of 3.11 J/cm3 at x = 0.08, which decreased with increasing PMN concentration.
Since the AFE-FE phase transition accompanied by volumetric expansion is sensitive to the applied stress, many researchers have utilized the mechanical confining effect to improve the energy storage properties.106,121,122) Patel et al.106) reported that the application of a mechanical stress reduces hysteresis loss and increases the AFE-FE transition by suppressing FE switching and initiating domain pinning. Gradual decreases in Pr and Pmax along with an increase in the EAFE-FE of (Pb0.96La0.04)(Zr0.90Ti0.1)0.99O3 ceramics were observed with an increase in the applied mechanical stress from 20 MPa to 300 MPa, which improved the Urec and η by 38% and 25%, respectively. On the other hand, the Urec of Pb0.99Nb0.02 [(Zr0.57Sn0.43)0.94Ti0.06]0.98O3 ceramics was enhanced by 23% (at 75 MPa) when a uniaxial stress was applied, whereas 17% (at 90 MPa) enhancement was observed for the application of a radial compressive stress.122) In another study, Young et al.121) demonstrated that mechanical self-confinement led to an improvement in the energy storage density of partially electroded Pb0.99Nb0.02[(Zr0.57Sn0.43)1-yTiy]0.98O3 (y = 0.050-0.064) ceramics. A phase-field model was used to simulate the coevolution of polarization and stress and revealed that the partially electroded sample (0.5A) exhibited a larger Pmax along with a higher as well as steeper AFE-FE transition than the fully electroded sample (1.0A), as shown in Fig. 7(c). The possible reasons for the enhanced properties such as larger polarization is due to the electrostatic fringe effect and the higher AFE-FE transition that is a result of the combined effect of mechanical self-confinement and relatively low defect concentration. The simulated stress distribution of the 0.5 A sample under the peak electric field (~ 70 kV/cm) is shown in Fig. 7(d), and the maximum stress that the sample experienced is approximately 30 MPa. All these effects resulted in a 9.2% enhancement in Urec (1.30 J/cm3 for the 0.5A sample and 1.19 J/cm3 for the 1.0A sample), though the η remained unchanged.
2.5.2. Lead-free AFE ceramics
Quite a few lead-free ceramics exhibit AFE behavior at RT; among them, (Bi, Na)TiO3 (BNT)-based138-151) and AgNbO3 (AN)-based152-156) ceramics have been extensively studied for energy storage applications. It is well known that BNT undergoes a structural transition from the rhombohedral phase to the tetragonal phase via an intermediate AFE-like phase at around 190°C (depolarization temperature, Td); however, this can be shifted to a lower temperature through compositional modification. In this regard, BT-modified BNT system has been extensively studied to stabilize the AFE phase by modulating the FE-AFE transition temperature.157) Li et al.143) modulated the antiferroelectricity and corresponding energy storage properties of 0.94Bi0.5-xNa0.5-xTiO3-0.06BaTiO3 (BNTx-BT) ceramics by tailoring the Bi/Na ratio. The composition corresponding to excess amounts of Bi3+ and/or deficiency in Na+ displayed a reduced Td with a well-developed AFE-like phase at RT, which resulted in double hysteresis loops with enhanced saturation polarization. As a result, a maximum energy storage density of 1.76 J/cm3 was achieved for x = 0.05 of the BNTx-BT ceramics.
Xu et al.144) reported that Pr and EC decreased while the BDS and ac-resistivity increased with the incorporation of La3+ in the BNT-BT matrix (i.e., [(Bi0.5Na0.5)0.93Ba0.07]1-xLax-TiO3, BNBLT7). In this study, two kinds of dopants, namely La2O3 (x = 0-0.04) and La(NO3)3 (x = 0.04) powders, were used. Since the ionic radius of La3+ (1.06 Å) is smaller than those of Bi3+ (1.36 Å) and Na+ (1.39 Å), shifting of diffraction peaks towards higher angles and a reduction in grain size were noticed with increasing La3+ concentration. Moreover, decreases in Pr from 44.90 μC/cm2 to 2.19 μC/cm2, EC from 34.0 kV/cm to 5.70 kV/cm, and an increase in the amount of the AFE-like phase of the BNBLT ceramics upon shifting the Td to below RT were observed. Consequently, improved energy storage densities of 1.09 J/cm3 (at 80 kV/cm) and 1.21 J/cm3 (at 100 kV/cm) were realized in La2O3 and La(NO3)3) doped BNT-BT samples, respectively. In another study, Li et al.147) reported that the incorporation of La3+ changed the crystal structure of BNT-BT ceramics from the FE rhombohedral phase to the AFE-like tetragonal phase and reduced the Pr and Pmax progressively. The La3+-modified BNT-BT ((Bi1/2Na1/2)0.94Ba0.06]La(1-x)ZrxTiO3, BNBLT6) ceramics exhibited a maximum strain of 0.53% and a strain coefficient of 707 pm/V for x = 0.03, while a larger Urec of 1.66 J/cm3 at 105 kV/cm was obtained for x = 0.05. Furthermore, addition of Zr increased the BDS and reduced the hysteresis loss of the BNBLT ceramics, thus, the energy storage properties were significantly improved.139,141) Recently, Kandula et al.149) investigated the effect of Nd3+ on the energy storage properties of BNT-BT AFE ceramics. The doping of 1 mol% Nd3+ in the BNT-BT matrix induced relaxor-like behavior (γ = 1.84) with improved permittivity (~ 3500@5kHz at Tm). With increasing temperature, both Urec and η gradually increased due to the increase in domain wall mobility and the ease of domain reorientation, which resulted in the large value of Urec of ~ 1.53 J/cm3 and an η of 93% at 73 kV/cm and 105°C.
Cao et al.138) demonstrated defect dipole-induced slim and double hysteresis loop behavior in the Mn2+-doped 0.7[0.94NBT-0.06BT]-0.3ST (NBBST) ternary system for enhanced energy storage properties. It was reported that the incorporation of the acceptor-type Mn2+ dopant for substituting Ti4+ resulted in the formation of oxygen vacancies to ensure charge balance and the production of defect dipoles (PD). According to the symmetry-conforming property of point defects, the symmetry of PD follows the crystal symmetry under the equilibrium condition. On application of an external electric field, PS tries to align in the direction of the field, but PD shows resistance to such a change (Fig. 7(e)). Moreover, PD can act as an internal electric field and help the new domains get back to their original state after the field is removed. Therefore, Mn2+-doped NBBST ceramics exhibit double hysteresis loop behavior with enlarged Pmax - Pr values (Fig. 7(f) and 7(g)). As a consequence, a maximum value of Urec (1.06 J/cm3 at 95 kV/cm) was obtained at 11 mol% Mn doping due to the large Pmax - Pr value (37 μC/cm2). Mishra et al.145) investigated the energy storage properties of BiFeO3 (BF) and K0.5Na0.5NbO3 (KNN) modified BNT-BT ceramics. In order to reduce the leakage current, 0.1 wt% of MnO2 was added to all the ceramics. The ternary system with the composition of 0.50BNTBT-0.50BFBT was optimal on account of its better energy storage properties (Urec = 0.77 J/cm3 and η = 67% at 50 kV/cm) compared to the other systems such as 0.25BNTBT-0.75BFBT and 0.75BNTBT-0.25BFBT. Further, doping of 1 mol% KNN to the optimized composition significantly increased the η from 67% to 90.3% at the cost of Urec (0.38 J/cm3). In addition, the 0.50BNTBT-0.50BFBT ceramics sintered using the SPS technique displayed a Urec of 1.3 J/cm3, which was 69% larger than that of the CS sample.
The other doped BNT system of (Bi, Na)TiO3-(Bi, K)TiO3 (BNT-BKT or BNKT) has been extensively studied owing to its enhanced piezoelectric and ferroelectric properties at the rhombohedral-tetragonal MPB. A few attempts have been made to improve the energy storage density properties of BNT-BKT ceramics by doping and/or forming ternary solid solutions with other perovskites.140,142,146,148) Zhao et al.140) reported that the addition of KNN improved the AFE phase stability and energy storage properties of 0.84Na0.5Bi0.5TiO3-0.16K0.5Bi0.5TiO3 (0.84BNT-0.16BKT) ceramics. As the x of (1-x)(0.84BNT-0.16BKT)-xKNN ceramics was increased (x = 0-0.15), the Td gradually decreased from 140°C to 75°C due to an increase in oxygen vacancies upon the substitution of the pentavalent Nb5+ (KNN) for the tetravalent Ti4+ (BNT-BKT) sites, which facilitated the increase in the contribution of the AFE phase; therefore, the P-E loops became slim and exhibited the double hysteretic behavior at RT. The ceramic of composition x = 0.09 exhibited the maximum Urec of 1.56 J/cm3 with an η of 64.5% and good temperature stability (ΔUrec/Urec@100 °C ≤ ± 20%) due to its uniform microstructure and slim double hysteresis loops. On the other hand, doping of a complex ion (Al0.5Nb0.5)4+ improved the BDS of the BNT-BKT ceramics.142) The substitution of Al3+ and Nb5+ for Ti4+ led to the creation of oxygen vacancies, as well as cation vacancies that compensated the charge imbalance, which increased the ability to withstand larger electric fields (~ 116 kV/cm). Moreover, (Al0.5Nb0.5)4+-containing BNT-BKT ceramics revealed reduced values of Pr, Pmax, and ionic and electronic conductivities. Therefore, the Urec continuously increased with (Al0.5Nb0.5)4+ concentration and reached a maximum value of 1.41 J/cm3 at 105 kV/cm when the doping was 8 mol%. Similarly, co-doping of Li+, Nb5+, Sr2+, and Ta5+ resulted in shifting the Td to below RT, relaxor-like behavior, reduced Pr, Pmax, and EC, and enhanced BDS of the BNT-BKT ceramics.148) All these effects made it possible to achieve the largest Urec of 1.60 J/cm3 at 90 kV/cm for ((Bi0.5[(Na0.8K0.2)0.90Li0.10]0.5)0.96Sr0.04)(Ti0.975Ta0.025Nb0)O3 (BNLKSTTN-0.10/0.04/0.025/0). In addition, BNLKSTTN-0.10/0.04/0.025/0 ceramics exhibited good bipolar fatigue endurances up to 107 polarization switching cycles and good thermal stability over the temperature range 20-200°C. In another study, Yu et al.146) reported that the incorporation of BiAlO3 (BA) changed the crystal structure of 0.75BNT-0.25BKT ceramics from the tetragonal phase to the pseudocubic phase and improved the AFE order. TEM observations of BA-doped 0.75BNT-0.25BKT ceramics revealed the existence of AFE features based on the appearance of ½(ooe) spots, which belong to the in-phase a0a0c+ oxygen octahedron tilt system with P4bm space group. The incorporation of BA destroyed the long-range FE order, which is evidenced by the decrease in Pr, Pmax, and EC and the appearance of pinched and slim hysteresis loops. At the optimal composition of 0.94(0.75BNT-0.25BKT)-0.06BA, the maximum value of 1.15 J/cm3 with an η of 73.2% at 105 kV/cm has been achieved.
Recently, Li et al.150) investigated the effect of doping of the AFE material NaNbO3 (NN) on the energy storage properties of 0.7Bi0.5Na0.5TiO3-0.3Bi0.2Sr0.7TiO3 (0.7BNT-0.3BST) AFE ceramics. With the incorporation of NN, increases in the grain size and BDS and decreases in ɛr, Pmax, and hysteresis loss were observed. Additionally, the doping of NN hindered the transformation of nanopolar clusters to macropolar clusters and resulted in slim double hysteresis loops even under large applied electric fields. Therefore, the existence of relaxor and AFE behaviors with large BDS enhanced the energy storage properties (Urec ~ 1.03 J/cm3 and η ~ 85.8% at 85 kV/cm) for 1 mol% NN-doped (0.7BNT-0.3BST) ceramics.
In the family of lead-free AFE ceramics, AgNbO3 (AN) is considered as a promising AFE candidate for energy storage applications owing to its inherently higher BDS and polarization properties.152-156) Tian et al.153) demonstrated the structure-property relation and energy storage properties of AN ceramics prepared via conventional sintering. Temperature-dependent FE measurements of the AN ceramics revealed the existence of two types of polar regions, one of which was stable up to 70°C and the other was stable up to 170°C. Earlier reports suggested that the transition at 170°C represents the freezing temperature of the antipolar dipoles of the Pbcm lattice. At RT, AN ceramics exhibited the typical AFE-like double hysteresis P-E loop with a Pmax of 40 μC/cm2 and a BDS of 175 kV/cm, which led to the realization of a maximum Urec value of ~ 2.1 J/cm3, though the η was too low (< 50%). Further, Zhao et al.152) considered MnO2 doping (0-0.3 wt%) to improve the η of AN ceramics. The addition of MnO2 reduced the sintering temperature, promoted densification, and improved the dielectric properties of AN ceramics. With increasing MnO2 concentration, the Pr decreased and both the switching fields increased monotonically, while the Pmax increased first and then decreased at the turning point of x = 0.1. The reduced Pr and ΔE and enhanced Pmax at x = 0.1 resulted in the Urec increasing from 1.6 J/cm3 to 2.5 J/cm3 and η from 37% to 57%. In addition to the improved Urec and η, Mn-doped AN ceramics displayed excellent temperature stability over the temperature range 20-180°C. Inspired by the above results, various dopants such as Ta2O5,154) WO3,155) and Bi2O3156) were introduced into AN ceramics to reduce the Pr and ΔE and increase the Pmax and switching fields, which succeeded in realizing larger values of Urec (2.6-4.2 J/cm3) and η (50-86%).
3. Conclusions and Future Prospects
The recent developments in pulsed power technology and hybrid electric vehicles propel the exploration of highly efficient energy storage materials having large energy storage and power densities, sustainability, and good fatigue lives. Dielectric ceramics are of particular interest for energy storage applications owing to their moderate energy storage density, large power density, fast charging/discharging, good mechanical and temperature stability, and good fatigue endurance. In this work, an overview of the technological developments towards improving the energy storage properties of various types of dielectric ceramics, including LD, PE, FE, RFE, and AFE materials have been presented. To achieve high energy storage properties in dielectric ceramics, various approaches such as chemical modification, grain refinement, core-shell structuring, the use of special sintering techniques, multilayered structures, interfacial engineering, mechanical confinement, self-confinement, and the fabrication of materials in which different phases coexist (near the MPB) have been utilized.
Even though significant progress has been made in realizing high energy storage properties, a few challenges remain to be resolved, including obtaining high ɛr, low tanδ, high BDS, low Pr, and high Pmax in the same dielectric material. Therefore, careful control of the processing parameters and design of a new dielectric material that can balance high polarization, low hysteresis, and high BDS are highly essential. Most of the studies have concentrated on improving the BDS rather than simultaneously improving both ɛr and BDS. However, the application of large electric fields to realize large Urec causes several safety issues. Moreover, for practical applications, high η is essential along with high Urec, therefore, the fabrication of high-permittivity nano-crystalline materials and/or materials in which the RFE and AFE phases coexist are highly recommended. In addition, the fabrication of multilayered structures consisting of appropriate combinations of high permittivity and low loss (or high BDS) layers is also a feasible route to high Urec as well as high η. Recently, Xu et al.158) reported their first-principle based theoretical predictions on the energy storage features of the rare-earth element Nd-modified BiFeO3 system, which is capable of achieving very high energy densities (100-150 J/cm3) and efficiencies (80-88%) even at practically available electric fields (2000-3000 kV/cm). Likewise, several theoretical studies combined with experimental strategies are required to design a material with enormous energy storage properties. Furthermore, the development of a standard evaluation system is required for replacing the currently used static and dynamic methods.