Ai, Liu, Zhang, Gong, He, and Zhang: Microwave Sintering of Graphene-Nanoplatelet-Reinforced Al2O3-based Composites
Abstract
In this study, we performed a microwave sintering (MWS) of Al2O3 ceramic and Al2O3-based composites with nominal contents of graphene nanoplatelets (GPLs) of 0.2, 0.4, 0.6, and 0.8 vol%. The GPL dispersion in N-methyl pyrroleketone was optimized to deagglomerate the GPLs without damaging their structure. Dense composites were then obtained by MWS at 1500°C for 30 min. The effects of different GPL contents on the phase compositions, microstructures, and mechanical properties of the composites were investigated. The microstructures of the composites became finer with the incorporation of the GPLs. The well-dispersed GPL fillers led to higher sintered densities in the composites. The optimal mechanical properties were achieved with 0.4 vol% GPLs. For this sample, the hardness, fracture toughness, and bending strength were 2000 kgf/mm2, 6.19 MPa·m1/2, and 365.10 MPa, respectively. The addition of GPL could improve the microstructure of the Al2O3 ceramic and has potential to improve the fracture toughness of the ceramics.
Key words: Microwave sintering, Al2O3-based composite, Graphene nanoplatelet, Microstructure, Mechanical properties
1. Introduction
Al 2O 3 ceramics have been widely used as high-speed cutting tools, dental implants, chemical and electrical insulators, resistance parts, and various coatings 1) owing to their excellent properties, such as high strength, hardness, temperature resistance, carrying-capacity corrosion resistance, and good chemical stability. 2-3) However, the fracture toughness of the pure Al 2O 3 ceramic material is very low. This significantly affects the work reliability and operation safety of the ceramic parts. 4) In order to overcome the limitations of the mechanical properties of Al 2O 3 ceramics, studies have been performed to prepare Al 2O 3-based composite ceramics by adding secondary phases. For example, Puchy et al. 5) used spark plasma sintering (SPS) to prepare Al 2O 3/carbon nanotube (CNT) nanocomposites and reported a fracture toughness of 4.14 MPa·m 1/2 at a CNT concentration of 5%. Ahmad et al. 6) employed hot-press sintering (HPS) to prepare Al 2O 3-based nanocomposites containing MgO, and reported that the optimal fracture toughness, flexural strength, and hardness were increased by 37%, 22%, and 20%, respectively, compared to those of monolithic alumina. Guo et al. 7) prepared zirconia-toughened alumina (ZTA) ceramics by mixing Al 2O 3 and yttria-tetragonal zirconia polycrystal (Y-TZP), isostatically pressed at 200 MPa and sintered at 1450°C for 2 h in air, whose fracture toughness and strength were up to 7.2 MPa·m 1/2 and 680 MPa, respectively. Li et al. 8) reported a novel approach to improve the hardness of a ZTA composite with a zirconia content of 20 vol%, whose Vickers hardness, flexural strength, and fracture toughness were 17.1 ± 2.5 GPa, 738 ± 88 MPa, and 4.2 ± 0.11 MPa·m 1/2, respectively. Parchoviansky et al. 9) reported the microstructures and mechanical properties of Al 2O 3/SiC nanocomposites prepared by HPS. The results indicated that the flexural strengths of the nanocomposites increased with the volume fraction of the silicon carbide particles; the maximum flexural strength was 655 ± 90 MPa for 20 vol.% SiC. Recently, graphene has attracted significant attention owing to its unique properties, 10,11) such as a large specific surface area, two-dimensional high-aspect-ratio sheet geometry, and outstanding mechanical properties. In addition, graphene was of significance for ceramic toughening. 12)
Composites reinforced with graphene nanoplatelets (GPLs), as a secondary additive, have been intensively developed. Celik et al. 13) reported an improvement in fracture toughness of 26.7% in graphene-based alumina-matrix nanocomposites. Bi et al. 14) prepared an Al 2O 3/GPL composite with a fracture toughness and bending strength of 6.4 MPa·m 1/2 and 541.9 MPa (corresponding to improvements of 48% and 16%), respectively. In addition to the performance improvements of the composites by adding secondary phases, the sintering method has a large influence on the properties of the composites. Different sintering methods yield different microstructures and mechanical properties of the composites.
Pressureless sintering (PS), HPS, SPS, and hot-isostatic-pressing sintering (HIP), are widely used to prepare Al 2O 3-based ceramic tool materials. 15) However, the samples prepared by these sintering methods have low comprehensive performances or require long sintering times and large costs. In order to improve the comprehensive performance and efficiency, and reduce the cost in the fabrication of Al 2O 3-based composites, microwave sintering (MWS) has been employed. 16-20)
Various factors affect the properties of Al2O3-based composites. Among them, except for the content of GPLs and sintering method, the dispersion of the GPLs in the Al2O3 matrix directly affects the mechanical properties of Al2O3-based composites. In order to provide uniformly dispersed GPLs in the Al2O3 matrix, appropriate amounts of suitable dispersants and additives should be added in the preparation of the Al2O3/GPL composites.
Therefore, in this study, GPLs were dispersed in an aqueous solution with the aid of a good dispersant, N-methyl pyrroleketone (NMP). The dispersed system was then incorporated into the Al2O3 matrix through ball-milling to provide uniformly dispersed GPLs for homogeneous Al2O3/GPL composites. Subsequently, the Al2O3/GPL composites were prepared by MWS. The effects of the GPL content on the microstructures and mechanical properties of the Al2O3/GPL composites were investigated in detail.
2. Experimental Procedure
2.1. Raw materials and preparation of Al2O3/GPL composite samples
Powders of Al2O3 (≥ 99.6%, Shandong ZBLP Ceramic Materials Co., Ltd.), graphene (GPLs, specific surface area: 500-1000 m2/g, Nanjing XFNANO Materials Tech Co., Ltd), and NMP (≥ 99%, AR, Tianjin Daming Chemical Reagent Factory) were used.
In order to obtain homogenized Al2O3/GPL composite samples, the dispersion of GPLs was performed in two steps. In the first step, GPLs were dispersed in a deionized water solution, while in the second step, they were distributed in the Al2O3 solution.
The GPLs were placed in a 500 mL glass beaker. A certain amount of water was then added into the glass beaker. The beaker was vibrated by ultrasonic waves for 20 min for pre-dispersion. NMP was then dissolved in deionized water in another beaker and stirred by hand for 3 min; subsequently, the GPL aqueous solution was poured into the aqueous solution of NMP and the pH was adjusted. The beaker was continuously vibrated by ultrasonic waves for 2 h, and the supernatant was centrifuged and dispersed to obtain a high-concentration stable GPL dispersion. In the first step for dispersion of the GPLs, the volume fractions of the GPLs in the aqueous solutions were set to 0, 0.2, 0.4, 0.6, and 0.8 vol.%.
In the second step, the GPL aqueous solution prepared in the first step was poured in the Al2O3 aqueous solution, which was vibrated by ultrasonic waves for 30 min. Subsequently, ball-milling was performed for 24 h at 200 r/min in anhydrous alcohol to obtain a homogenous mixture. The mixtures were then dried at 90°C; the dry powders were sieved through a 200-mesh sieve, yielding the GPL/Al2O3 composite powders. Finally, the composite powders were compacted with a metal mold under a pressure of 80 MPa and cold-isostatically pressed at 200 MPa. The shaped GPL/Al2O3 composites underwent MWS. After sintering, the samples were ground and polished using a SiC paper and chromium oxide suspension.
2.2. Characterization
Before the test, all the sintered samples were ground and polished. The relative densities of the samples were measured by the Archimedes’ method. Their hardness values were measured using a Vickers hardness tester with a loading of 20 kg. According to the national standards GB/T6569-2006 and GB/T23806-2009, the three-point bending and unilateral pre-cracked beam methods are used to determine the flexural strength and fracture toughness, respectively. The corresponding loading rates in the Electronic Universal Test Machine were 0.5 and 0.05 mm/min, respectively. X-ray diffraction (XRD, D8 Advance) was used for phase identification. The morphologies of the GPLs were observed by field-emission scanning electron microscopy (SEM, Nova Nano SEM450).
3. Results and Discussion
3.1. Phase compositions and microstructures of the Al2O3/GPL composites
The XRD patterns of the sintered composites are shown in Fig. 1. The compositions of the composite ceramics are similar. Two characteristic peaks are observed at 26.5° and 42.9°, corresponding to the G (002) and G (100) peaks of the GPLs, respectively, which demonstrate the existence of GPLs in the composites. In addition, the Bragg peak of Al 2O 3 is observed. All the diffraction peaks are observed in the composite materials; no other secondary phases 21,22) are generated at the different GPL contents, which indicates the excellent chemical compatibility between the GPLs and Al 2O 3 ceramic particles in the high-temperature MWS. However, owing to the low content of GPLs, the XRD peaks of the GPLs in the sintered samples are not very intense.
Figure 2 illustrates the SEM morphologies of the fractured surfaces of the sintered samples with and without GPLs. Fig. 2(a) shows few larger particles in the sintered specimen of pure Al 2O 3; the structure of the tissue is loose with few pores. Figs. 2(b) and 2(c) show SEM micrographs of the sintered samples with 0.2- and 0.4-vol.% GPLs, respectively. According to Figs. 2(b) and 2(c) and average Al 2O 3 particle size statistics in Figs. 2(a)-(c), after the addition of the GPLs, the grains of the sintered samples seem slightly smaller than those of the pure Al 2O 3 ceramic material ( Fig. 2(a)). This could be explained as the GPLs embedded in the grain boundaries (white circles) can stop grain growth and prevent the movement of grain boundaries, referred to as pinning effect of the GPLs. This reveals the good distribution of the GPLs. Another factor may be that the GPLs exhibit large thermal conductivities, 23) so that the GPLs not only promote a uniform sintering temperature, but also accelerate the cooling. Simultaneously, the temperature distributions in the samples are uniform owing to the coupling between the whole specimen and electromagnetic wave in the MWS. However, when the GPL content reaches 0.6 vol.%, a significant agglomeration of GPLs occurs, as shown in Fig. 2(d). When the GPL content is higher (0.8 vol.%), an abnormal grain growth is observed, as shown in Fig. 2(e), as the too high content of GPLs hinders their uniform distribution in the composites, leading to the agglomerated GPLs. This is not conducive to the densification of the composites, as it reduces the relative density. This implies the formation of pores in the composites, which also provides space for the growth of grains. The aggregation of the GPLs leads to pinning effect reduction, which in turn leads to an increase in grain size.
Figure 3 shows SEM morphologies and energy-dispersive spectra (EDS) of fractured surfaces of the Al 2O 3/0.4-vol.%-GPL composite. Fig. 3(c) shows that Spectrum 1 contains only C, O, and Al elements; the carbon content is relatively high, which is attributed to the GPLs. This could be explained as only graphene was used as a carbon material to prepare the Al 2O 3-based composite ceramics and thus the content of graphene is high in the test area. Fig. 3(b) shows that flaky GPLs exist between adjacent Al 2O 3 grains, which are evenly bent along the Al 2O 3 grain boundary. The anchoring effect emerges when the GPLs are wrapped around the Al 2O 3 grain boundaries and bundled together. This effect can provide a higher energy to resist the removal of GPLs from the Al 2O 3 ceramic matrix, which can enhance the interfacial adhesion. Therefore, the fracture toughness can be improved owing to the enhanced interfacial friction. Therefore, this toughening mechanism is better than the traditional mechanism. 24)
3.2. Mechanical properties of the Al2O3/GPL composites
Figure 4 shows the relative densities of the Al 2O 3/ x-vol.%-GPL ( x = 0, 0.2, 0.4, 0.6, 0.8) composite ceramics prepared by the MWS at 1500°C for 30 min. Compared with that of the Al 2O 3 ceramic, the relative densities of the Al 2O 3/GPL composite ceramics are significantly decreased upon the addition of the GPLs. This occurs mainly as the addition of the GPLs hinders the complete densification of the Al 2O 3-based composites during the consolidation process. However, the relative densities of the Al 2O 3/GPL composites increase with the GPL volume content, at low volume contents of the GPLs. The maximum relative density is reached at a GPL content of 0.4 vol.%. The relative densities of the Al 2O 3/GPL composites decrease with further increase in the GPL volume content. This could be explained as the GPLs can be evenly distributed in the composites at low volume contents of the GPLs (≤ 0.4 vol.%) so that fewer pores are formed in the composites in the MWS, which is favorable to promote the sintering. Therefore, the relative densities of the composites increase. However, when the content of GPLs is higher (> 0.4 vol.%, e.g., 0.6 and 0.8 vol.%), the GPLs easily agglomerate. Therefore, more pores easily form, which reduces the relative densities of the composites in the MWS.
The effects of the volume content of GPLs on the hardness values of the Al 2O 3/GPL composites are shown in Fig. 5. The Vickers hardness rapidly decreases, varying with the addition of the GPLs, from 2550 kgf/mm 2 for the pure Al 2O 3 ceramic to 1610 kgf/mm 2 for the Al 2O 3/0.8-vol%-GPL composite. When the content of GPLs is smaller than 0.4 vol.%, the Vickers hardness values of the Al 2O 3/GPL composites are increased; however, an excessive amount of GPLs leads to a reduced Vickers hardness. When the content of GPLs is 0.4 vol.%, the Vickers hardness reaches the maximum value of 2000 kgf/mm 2. The Vickers hardness and relative density exhibit the same trend. According to the Hall-Petch relationship, the hardness of a material is inversely proportional with the grain size. As the Al 2O 3 grain growth was inhibited and the grain size distribution was uniform after the addition of the GPL powder (smaller than 0.4 vol.%) (see Figs. 2(a) and 2(b)), the Vickers hardness values of the samples could be improved to some extent. In addition, the low modulus of elasticity of the GPLs could decrease the Vickers hardness of the Al 2O 3/GPL composites. 25)
However, Figs. 6 and 7 show that both bending strength and fracture toughness values of the Al 2O 3/GPL composites initially increase and then decrease with the increase in the content of GPLs. Compared with those of the pure Al 2O 3 ceramic, the fracture toughness and bending strength values of the Al 2O 3/GPL composites are significantly improved upon the addition of the GPLs. When the content of GPLs is 0.4 vol.%, both fracture toughness and bending strength reach their peak values. The maximum fracture toughness and bending strength of the Al 2O 3/GPL composites are 6.19 MPa·m 1/2 and 365.10 MPa, approximately 79% and 12% higher than those of the pure Al 2O 3 ceramic, respectively. Although both fracture toughness and bending strength decrease when the content of GPLs is larger than 0.4 vol.%, they are higher than those of the pure Al 2O 3 ceramic. This phenomenon may be attributed to the pores in the composite, which are believed to be the origins of cracks and weakening of the boundaries of the ceramic matrix. 26) Consequently, the optimal mechanical properties can be obtained with 0.4 vol.% GPLs. For this sample, the relative density, micro-hardness, fracture toughness, and bending strength are 98.8%, 2000 kgf/mm 2, 6.19 MPa·m 1/2, and 365.10 MPa, respectively. The relative density is decreased by approximately 0.4%, the hardness is decreased by approximately 22.5%, while the bending strength and fracture toughness are increased by approximately 12% and 79%, respectively, compared to those of the pure Al 2O 3 ceramic.
4. Conclusions
In this study, Al2O3/GPL composites with different amounts of GPLs were prepared by MWS at 1500°C for 30 min. We can summarize the following conclusions:
The GPLs could be well distributed in NMP. In addition, there was good chemical compatibility between GPLs and Al2O3 ceramic particles in the MWS and the microstructure was improved. The added GPLs could suppress the growth of Al2O3 grains. However, for a larger content of GPLs (larger than 0.4 vol.%), the agglomeration of GPLs was significant. With the increase in the content of GPLs, the fracture toughness and bending strength values of the samples initially increased and then decreased. The Al2O3/GPL composite with 0.4 vol.% GPLs exhibited the best mechanical properties. Its relative density, microhardness, fracture toughness, and bending strength were 98.8%, 2000 kgf/mm2, 6.19 MPa·m1/2, and 365.10 MPa, respectively. Compared with those of the Al2O3 ceramic, the bending strength and fracture toughness increased by approximately 12% and 79%, while the hardness and relative density decreased by approximately 22.5% and 0.4%, respectively. In general, GPLs have the potential to improve Al2O3-based ceramic matrix composites and to provide various light and strong ceramics for engineering applications. Crack deflection, crack bridging, and pull-out of GPLs were observed in this study, which were considered the main factors for toughening of the Al2O3-based composite materials.
Acknowledgments
This study was financially supported by Natural Science Key Foundation of the Jiangxi Provincial Education Department (GJJ150698) and Natural Science Foundation of the Jiangxi Provincial Education Department (GJJ160688).
Fig. 1
XRD of the sintered Al2O3/x-vol.%-GPL composite ceramics with x of (a) 0, (b) 0.2, (c) 0.4, (d) 0.6, and (e) 0.8 vol.%. 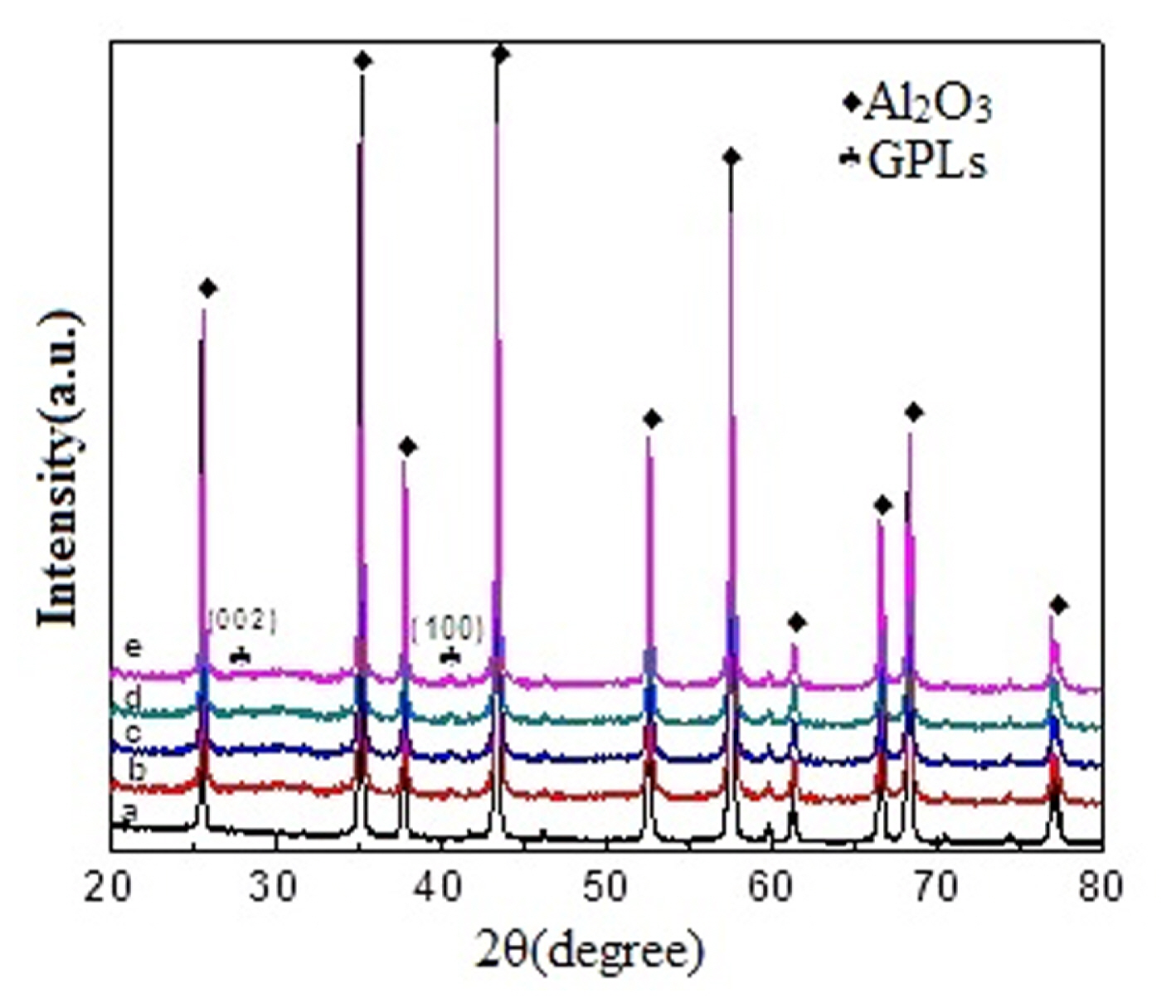
Fig. 2
SEM morphologies of fractured surfaces of the sintered samples: (a) pure Al2O3 and Al2O3/GPL composites with x of (b) 0.2, (c) 0.4, (d) 0.6, and (e) 0.8 vol.%. 
Fig. 3
SEM morphologies and EDS of fractured surfaces of the Al2O3/0.4-vol.%-GPL composite. 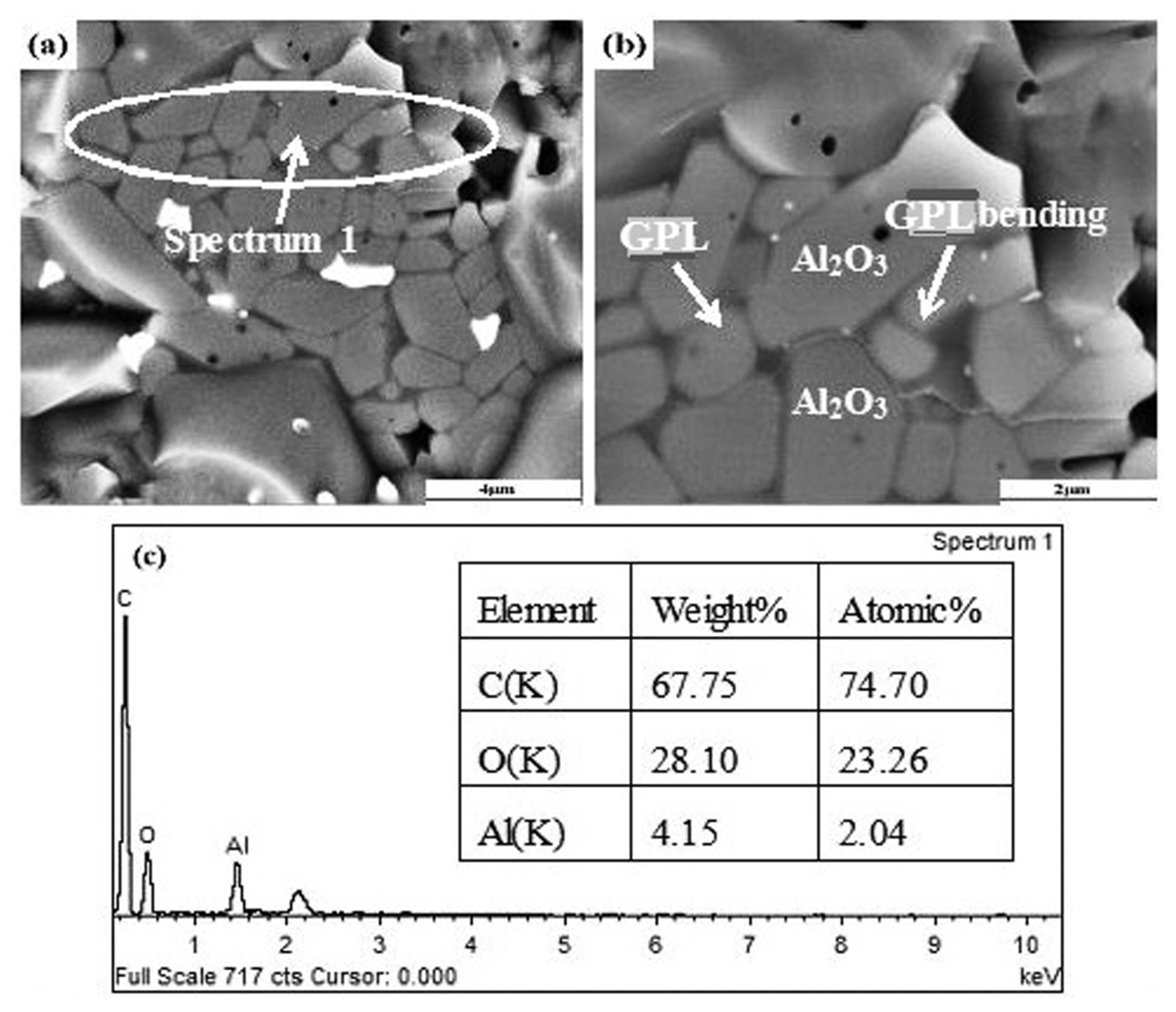
Fig. 4
Relative densities of the Al2O3/x-vol.%-GPL (x = 0, 0.2, 0.4, 0.6, 0.8) composite ceramics. 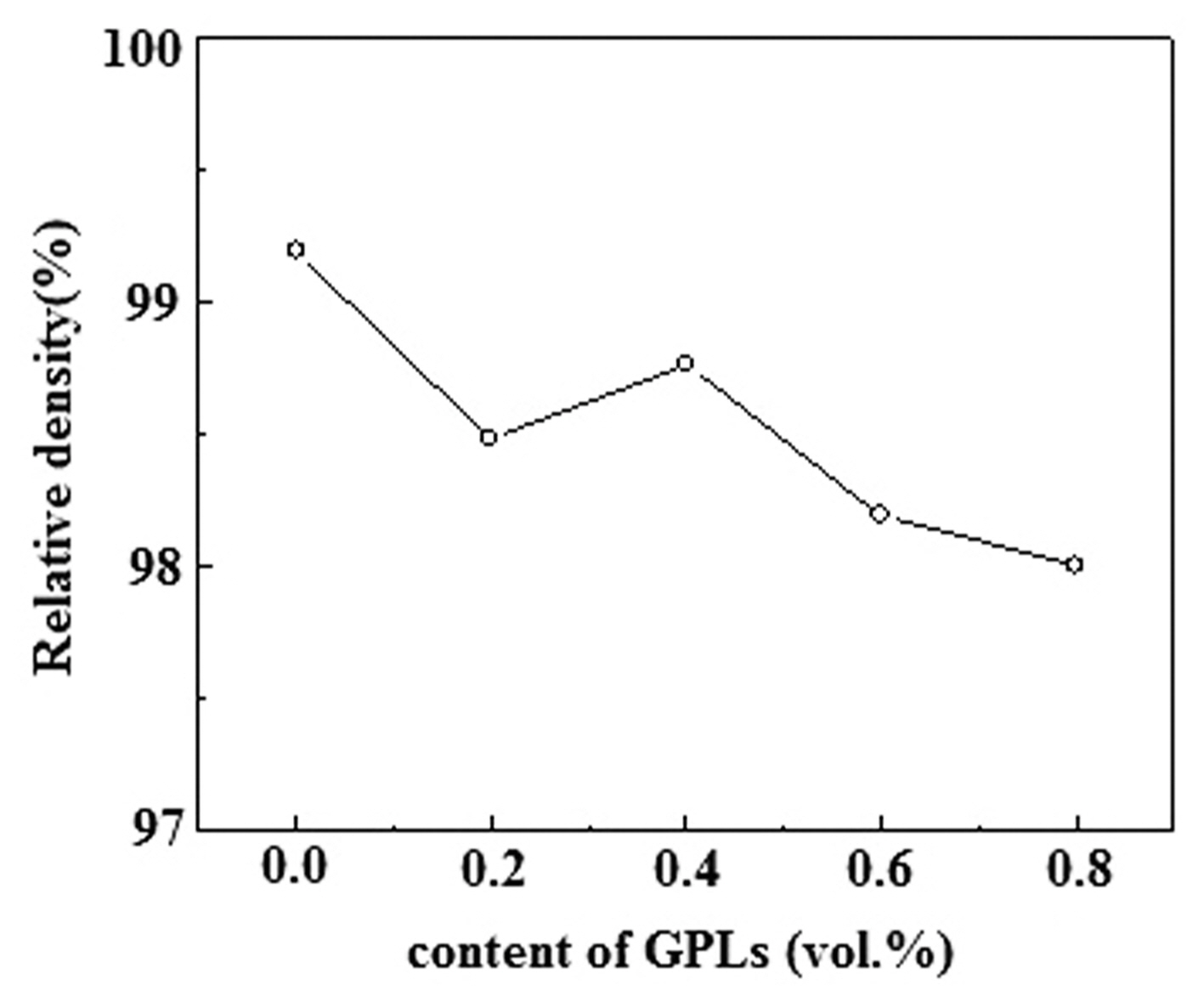
Fig. 5
Vickers hardness values of the Al2O3/x-vol.%-GPL (x = 0, 0.2, 0.4, 0.6, 0.8) composite ceramics. 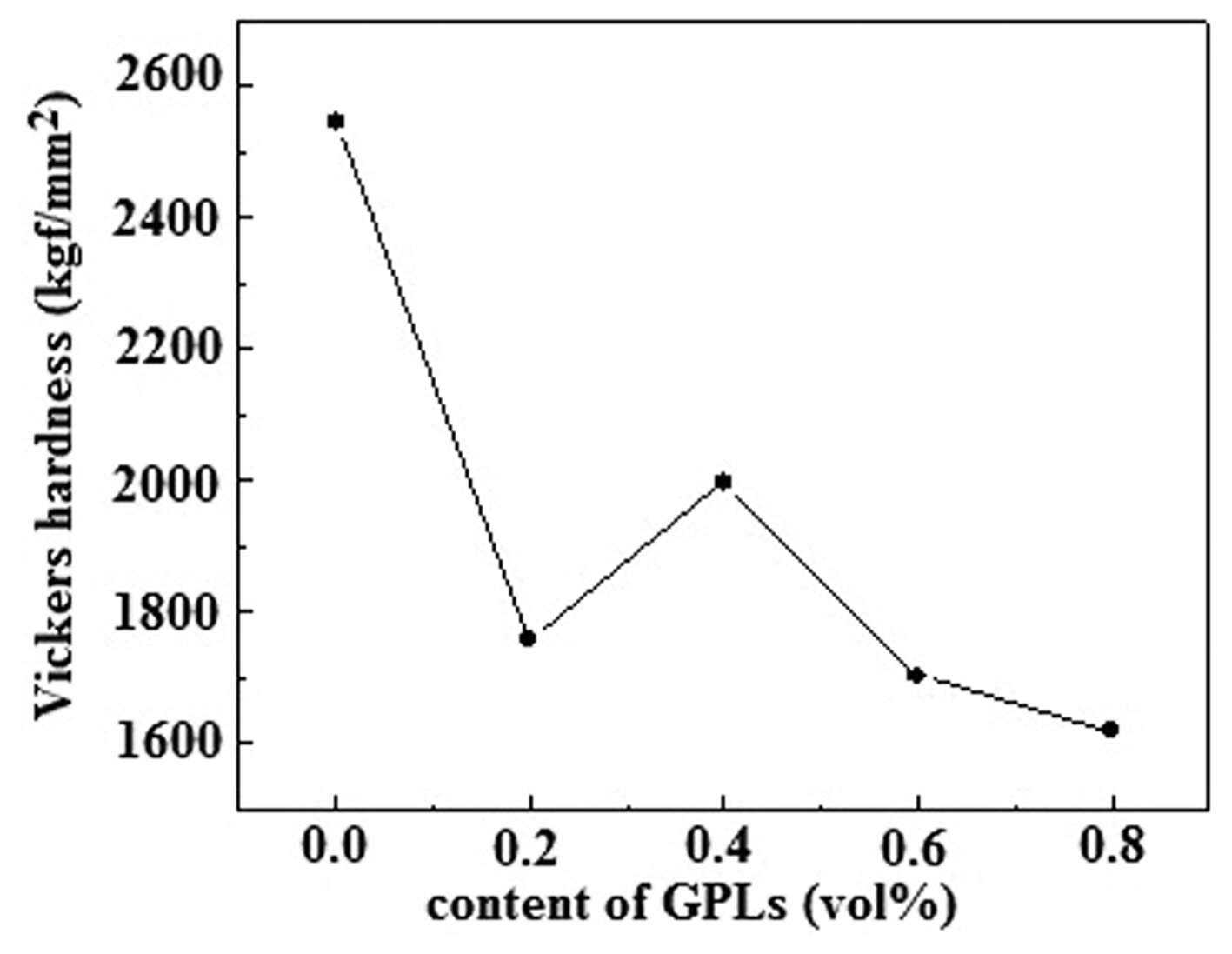
Fig. 6
Bending strengths of the Al2O3/x-vol.%-GPL (x = 0, 0.2, 0.4, 0.6, 0.8) composite ceramics. 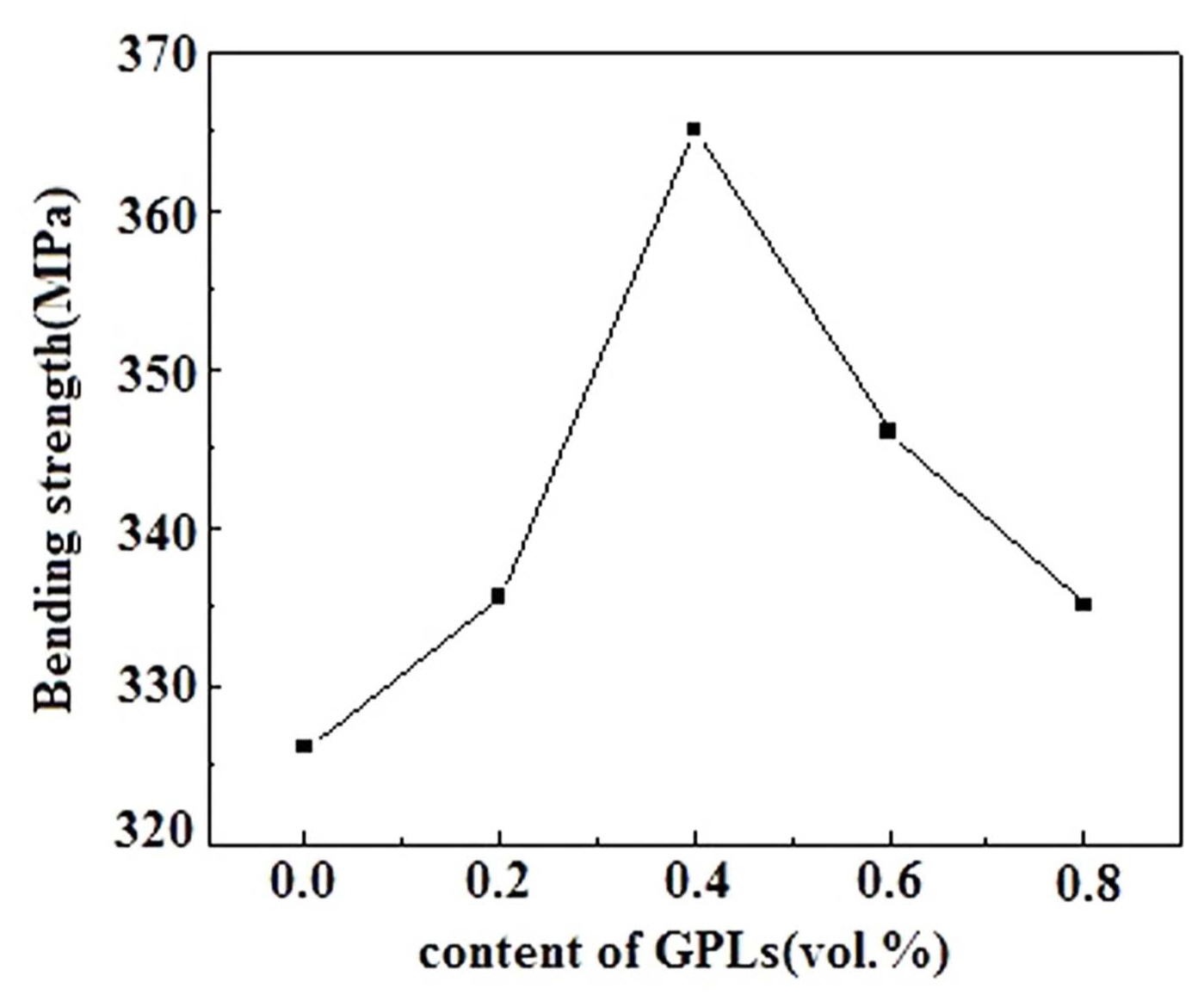
Fig. 7
Fracture toughness values of the Al2O3/x-vol.%-GPL (x = 0, 0.2, 0.4, 0.6, 0.8) composite ceramics. 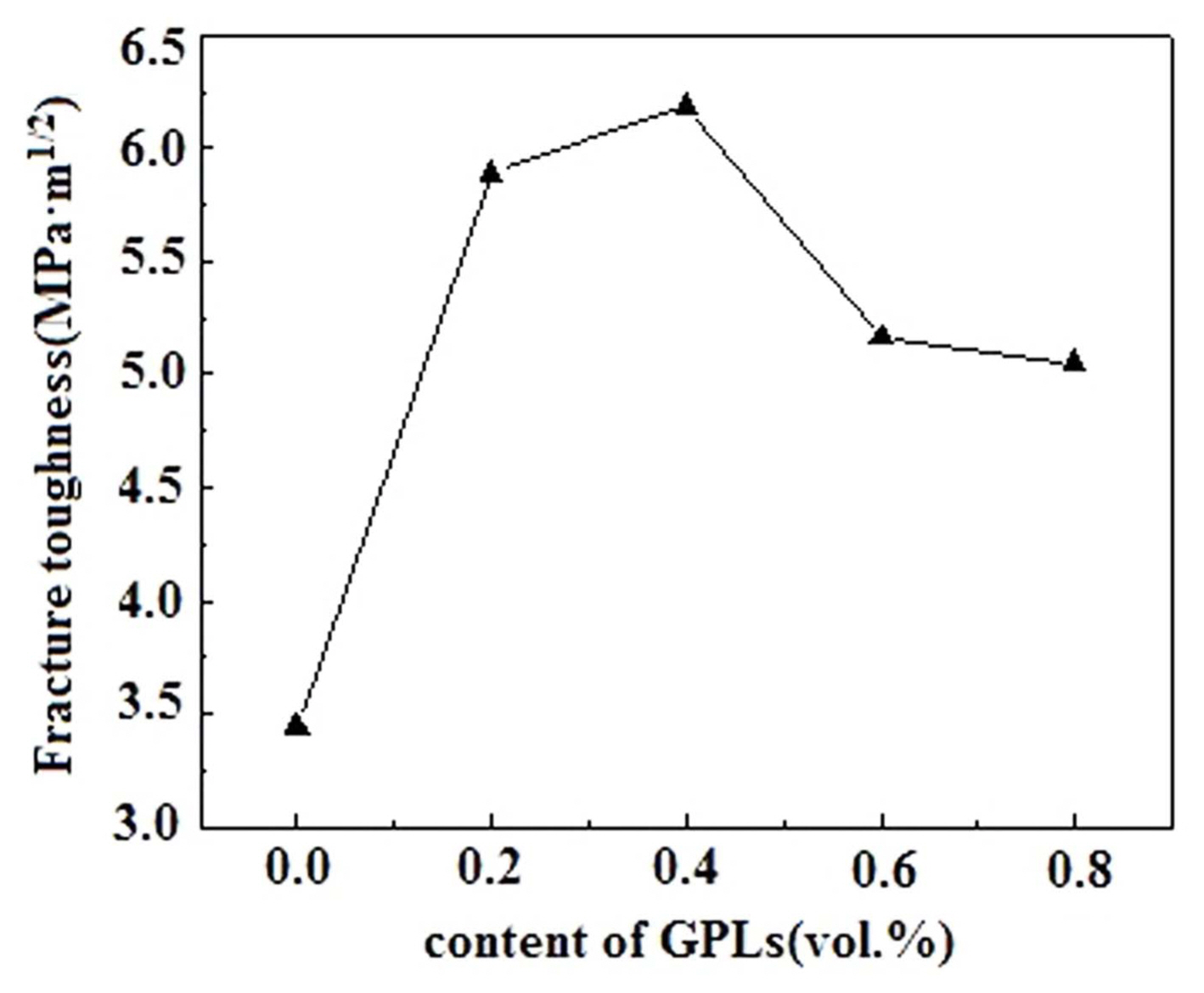
REFERENCES
1. I. Ahmad, HZ. Cao, HH. Chen, H. Zhao, A. Kennedy, and YQ. Zhu, “Carbon Toughened Aluminium Oxide Nanocomposite,” J Eur Ceram Soc, 30 [4] 865-73 (2010).  2. JW. Zheng, DM. Gao, L. Qiao, Y. Ying, WC. Li, LQ. Jiang, and SL. Che, “Influence of the Cu2O Morphology on the Metallization of Al2O3 Ceramics,” Surf Coat Technol, 285 249-54 (2016).  3. AK. Swarnakar, SG. Huang, OV. Biest, and J. Vleugels, “Ultrafine Al2O3-B4C Composites Consolidated by Pulsed Electric Current Sintering,” J Alloys Compd, 499 [2] 200-5 (2010).  4. ZP. Xie, WJ. Xue, and HB. Chen, “Research of Mechanical and Thermal Properties of Al2O3 at Cryogenic Temperatures,” Rare Met Mater Eng, 40 [S1] 597-99 (2011).
5. V. Puchy, P. Hvizdos, J. Dusza, F. kovac, F. Inam, and MJ. Reece, “Wear Resistance of Al2O3-CNT Ceramic Nanocomposites at Room and High Temperatures,” Ceram Int, 39 [5] 5821-26 (2013).  6. I. Ahmad, M. Islam, MA. Dar, F. Xu, and SI. Shad, “Magnesia Tuned Multi-Walled Carbon Nanotubes-Reinforced Alumina Nanocomposites,” Mater Charact, 99 210-19 (2015).  7. RS. Guo, DL. Guo, D. Zhao, ZF. Yang, and YR. Chen, “Low Temperature Aging in Water Vapor and Mechanical Properties of ZTA Ceramics,” Mater Lett, 56 [6] 1014-18 (2002).  8. YL. Li, HE. Kim, and YH. Koh, “Improving the Surface Hardness of Zirconia Toughened Alumina (ZTA) Composites by Surface Treatment with a Boehmite,” Ceram Int, 38 [4] 2889-92 (2012).  9. M. Parchoviansky, D. Galusek, J. Sedlacek, P. Svancarek, M. Kasiarova, J. Dusza, and P. Sajgalik, “Microstructure and Mechanical Properties of Hot Pressed Al2O3/SiC Nanocomposites,” J Eur Ceram Soc, 33 [12] 2291-98 (2013).  10. J. Liu, HX. Yan, MJ. Reece, and K. Jiang, “Toughening of Zirconia/Alumina Composites by the Addition of Graphene Platelets,” J Eur Ceram Soc, 32 [16] 4185-93 (2012).  11. LK. vetkova, A. Duszova, P. Hvizdos, J. Dusza, P. Kun, and C. Balazsi, “Fracture Toughness and Toughening Mechanisms in Graphene Platelet Reinforced Si3N4 Composites,” Scr Mater, 66 [10] 793-96 (2012).  12. DX. Li, ZH. Yang, DC. Jia, XM. Duan, PG. He, J. Yu, and Y. Zhou, “Spark Plasma Sintering and Toughening of Graphene Platelets Reinforced SiBCN Nanocomposites,” Ceram Int, 41 [9] 10755-65 (2015).  13. Y. Celik, A. Celik, E. Flahaut, and E. Suvaci, “Anisotropic Mechanical and Functional Properties of Graphene-Based Alumina Matrix Nanocomposites,” J Eur Ceram Soc, 36 [8] 2075-86 (2016).  14. JQ. Bi, “A Method of Preparation of Alumina Ceramics Reinforced by Graphene Nanosheets,” China Patent, 201310018280.4. 4-14 2013.
15. ZB. Yin, JT. Yuan, ZH. Wang, HP. Hu, Y. Cheng, and XQ. Hu, “Preparation and Properties of an Al2O3/Ti(C,N) Micro-Nano-Composite Ceramic Tool Material by Microwave Sintering,” Ceram Int, 42 [3] 4099-106 (2016).  16. HY. Yang, XG. Zhou, JS. Yu, HL. Wang, and ZL. Huang, “Microwave and Conventional Sintering of SiC/SiC Composites: Flexural Properties and Microstructures,” Ceram Int, 41 11651-54 (2015).  17. S. Manivannan, A. Joseph, PK. Sharma, KCJ. Raju, and D. Das, “Effect of Microwave and Conventional Sintering on Densification, Microstructure and Dielectric Properties of BZT-xCr2O3 Ceramics,” Ceram Int, 41 [9] 10923-33 (2015).  18. MO. ghbazi, and O. Mirzaee, “Microwave Versus Conventional Sintering: A Review of Fundamentals, Advantages and Applications,” J Alloys Compd, 494 [1-2] 175-89 (2010).  19. A. Presenda, MD. Salvador, L. Felipe, P. Foix, R. Moreno, and A. Borrell, “Effect of Microwave Sintering on Microstructure and Mechanical Properties in Y-TZP Materials Used for Dental Applications,” Ceram Int, 41 [5] 7125-32 (2015).  20. F. Zuo, A. Badev, S. Saunier, D. Goeuriot, R. Heuguet, and S. Marinel, “Microwave Versus Conventional Sintering: Estimate of the Apparent Activation Energy for Densification of α-Alumina and Zinc Oxide,” J Eur Ceram Soc, 34 [12] 3103-10 (2014).  21. M. Khenfouch, U. Buttner, and M. Baitoul, “Systhesis and Characterization of Mass Produced High Quality Few Layered Graphene Sheets via a Chemical Method,” Graphene, 3 7-13 (2014).  22. XY. Yang, “Performance and Mechanism study of ZnO Graphene Hybid Material (in Chinese),” 19-40 M S Thesis, University of Science and Technology of China, Hefei2014.
23. AA. Balandin, S. Ghosh, W. Bao, I. Calizo, D. Teweldebrhan, and F. Miao, “Superior Thermal Conductivity of Single-Layer Graphene,” Nano Lett, 8 [3] 902-7 (2008).  24. B. Yazdani, and I. Ahmad, “Graphene and Carbon Nanotube (CNT)-Reinforced Alumina Nanocomposites,” J Eur Ceram Soc, 35 [1] 179-86 (2015).  25. Y. Celik, A. Celik, and E. Flahaut, “Anisotropic Mechanical and Functional Properties of Graphene-Based Alumina Matrix Nanocomposites,” J Eur Ceram Soc, 36 2075-83 (2016).  26. J. Liu, HX. Yan, and K. Jiang, “Mechanical Properties of Grapheme Platelet-Reinforced Alumina Ceramic Composites,” Ceram Int, 39 [6] 6215-21 (2013). 
|
|


















