AbstractHydroxyapatites (HAps) were synthesized using the powdered waste of fishery products, i.e., fish scales and crab shells, as starting materials. HAp was synthesized by a wet-chemistry method followed by calcination at 600 and 800°C. Calcined crab-shell powder revealed a single HAp phase and fine powder, while calcined fish-scale powder showed a β-TCP secondary phase, even at the higher calcination temperature. Dense HAp pellets were obtained from the crab-shell powder by spark plasma sintering at 1000°C for 10 min under applied pressures of 40 and 80 MPa in a vacuum state, giving sample densities of 2.93 and 3.06 g/cm3, respectively. The estimated grain size of HAp was 448 ± 96 and 283 ± 59 nm for applied pressures of 40 and 80 MPa, respectively. In contrast, the HAp obtained using the pressureless sintering technique showed excessive grain growth without further densification.
1. IntroductionA biomaterial can be defined as a bio-based material or value-added biological material utilized for food, feed, fuel, pharmaceutical, or nutraceutical application. By definition, a biomaterial is also a bioproduct. A further definition is that a biomaterial is a non-viable material that is commonly used for the augmentation of organs or as medical devices. All materials made from metals, polymers, ceramics, or bio-based materials can be utilized as biomaterials, provided they comply with the biocompatibility requirements.1) Biocompatibility means that the material must be able to interact with living systems without harming its surrounding biological systems.
HAp of the chemical formula Ca10(PO4)6(OH)2 is the main constituent component of hard tissue, such as bone and teeth, in the human body, and has superior biocompatibility. 2) HAp can be synthesized from natural calcium-rich resources and employed for hard tissue regeneration, bone scaffolds, functional particles, and ceramic membranes for protein or lipid purifications.3-5)
Traditionally, HAp is synthesized by mixing the calcium precursor with a phosphate precursor. Some studies utilized wet-chemistry methods, such as sol-gel and hydrothermal, to synthesize HAp.6-9) In addition to the existing chemical methods, HAp can also be synthesized using waste materials such as crab shells, sea shells, fish scales, fish bones, and coral,8,10-19) because the major elements of these natural materials are similar to those of HAp. As a maritime country, Indonesia has numerous fishery industries with abundant by-products such as fish scales and crab shells. To date, the utilization of these by-products for advanced products has been limited. Therefore, the utilization of these byproducts as raw materials for the synthesis of HAp may increase the economic value of these waste materials.
Mondal et al.16) reported the sintering of HAp from fish scales at 1200°C with a pressureless sintering technique. To decrease the sintering temperature, the use of pressure-assisted sintering is imperative. For instance, the typical sintering temperatures of SiC or its composites are 2100 - 2500°C and can be decreased to 1750°C using a hot-pressing vacuum furnace20) or spark plasma sintering.21) Thus, dense and transparent HAps were successfully fabricated by spark plasma sintering at a relatively low temperature compared to pressureless sintering techniques.22-26) However, only a few records have been found on the pressure-assisted sintering of HAp from natural resources or waste material.
To the authors’ knowledge, there are no detailed studies that have investigated the synthesis and sinterability of HAp from fishery by-products. Thus, the aim of this study is to obtain HAp from the waste of fishery products, as well as to investigate the role of sintering technique, such as pressureless sintering and pressure-assisted sintering, i.e., spark plasma sintering. We used fish scales and crab shells as the starting materials. The data for the phase analysis, microstructure, density, and mechanical properties are presented and discussed in detail to obtain a better understanding of the role of pressure in the properties of sintered HAp.
2. Experimental ProcedureFish scales (Nemipterus nematophorus) and crab shells (Portunus pelagicus) as precursors for this study were obtained from Kelola Mina Laut Company, Indonesia. Crab shells and fish scales were boiled in water for 30 min, and then washed using distilled water at room temperature. The as-washed crab shells and fish scales were stirred in a 1% NaOH solution for 2 h and left overnight. Prior to sterilization in an autoclave at 110°C for 5 h, the crab shells and fish scales were washed with distilled water. Herein, CaO powder was obtained by calcining the crab shells and fish scales at 1000°C for 5 h. Then, approximately 50 and 90 g of calcined crab shells and fish scales, respectively, were dissolved in 200 mL of distilled water. The suspensions were then reacted with 0.21 and 0.04 mol of 85% H3PO4, respectively. The pH was maintained at 10 during stirring for 1 h at 60°C. After 24 h of aging at 27°C, the precipitate was filtered and dried at 110°C for 5 h. The dried samples were calcined at 600 and 800°C for 5 h in an air atmosphere to obtain HAp powder. The HAp powder obtained from crab shells (named CHAp) was poured into a 15 mm graphite die and sintered at 1000°C for 10 min at uniaxial pressures of 40 and 80 MPa in a vacuum state via spark plasma sintering with heating and cooling rates of 20°C min−1. The sintered HAp was black owing to carbon contamination from the graphite die. To eliminate the carbon, the sintered HAp was heat-treated at 800°C for 2 h in an air atmosphere. For comparison purposes, CHAp was also sintered using a pressureless sintering method at 1000, 1100, and 1200°C for 5 h in the ambient condition.
The element compositions of the calcined crab shells and fish scales were determined using X-ray fluorescence (XRF: Minipal 4, PANalytical, The Netherlands). The phases of the calcined powder as well as sintered pellets, were investigated using room-temperature X-ray diffraction (XRD: X’Pert-PRO MPD, PANalytical, The Netherlands) with the Cu-Kα line. The densities of the sintered samples were measured using the Archimedes principle. The morphologies of the powder particles and sintered bodies were observed using scanning electron microscopy (SEM: S-4800, Hitachi, at 10 kV and 10 μA). The average particle and grain sizes were estimated from the SEM images and analyzed statistically.
3. Results and Discussion
Table 1 shows the elemental compositions of the calcined crab shells and fish scales sintered at 1000°C for 5 h. As shown, the major element is Ca; the crab shells possess a higher Ca content (93.78%) than fish scales (82.31%). In contrast, the fish scales have a higher phosphor content than its counterpart. These results indicate that both fish scales and crab shells are potential candidates as raw materials for HAp production. However, further work is required to eliminate their impurities.
Figure 1 shows the XRD patterns of HAp powder from crab shells (CHAp) and fish scales (FHAp) after calcination at 600 and 800°C for 5 h. As shown in Figs. 1(a) and (b), only hydroxyapatite (Ca10(PO4)6(OH)2) is observed without any trace of secondary phases for CHAp, even at low calcination temperatures (600°C). The crystallization of CHAp is improved with higher calcination temperature, as shown in Fig. 1(b). On the contrary, secondary phases (β-tricalcium phosphate/β-TCP/Ca3(PO4)2) are observed for both FHAp powders (600 and 800°C), as shown in Figs. 1(c) and (d). At 800°C, the peaks of β-TCP are still observed, even though HAp phase becomes dominant. It is believed that the β-TCP phase would transform into the HAp phase with further calcination at higher temperature, since β-TCP is an unstable phase.27) CHAp has a single phase owing to its finer particle size, as shown in Fig. 2(b). The finer particle size indicates a higher surface area, which leads to higher activity and ease of forming the single phase. Thus, HAp can be formed at lower temperature without the formation of the secondary phase, as displayed in Fig. 1(a).
Figure 2 presents the SEM images of FHAp and CHAp after calcination at 800°C for 5 h. It is very clear that CHAp possesses a finer morphology than FHAp. FHAp and CHAp have a flat and round shape with a particle size of > 1 μm and 105 ± 28 nm, respectively. The particle properties of CHAp show the typical results of the wet chemistry method, i.e., precipitation. Yet, FHAp reaches an unexpected particle size, which is likely due to the existing impurity of FHAp. Since the methods of synthesizing HAp from FHAp and CHAp are identical, this suggests that the difference in particle size of these powders is likely due to impurity content. As shown in Table 1, FHAp only has 82.31% of Ca compared to the 93.78% of Ca in CHAp. Moreover, the secondary phase is observed in FHAp, as shown in Fig. 1; it was not found in CHAp.
Figure 3 shows the SEM images of the fractured surface of sintered HAp. As shown in Figs. 3(a), (b), and (c), the pressureless sintering of CHAp shows no sign of densification. Although necking is observed in Fig. 3(c), pressureless sintering yields no further densification mechanisms in CHAp. It should be noted that the CHAp particle size grows significantly at a sintering temperature of 1200°C when the pressureless sintering method is used. In contrast, a dense CHAp microstructure is obtained using the spark plasma sintering method, as shown in Figs. 3(d) and (e). These pellets were sintered at 1000°C for 10 min, indicating the effectiveness of spark plasma sintering in obtaining dense HAp in a short time and at a lower temperature in comparison with the conventional sintering method. Under the applied pressures of 40 and 80 MPa, the density of CHAp sintered at 1000°C reaches 2.93 and 3.06 g/cm3, respectively, which corresponds to relative densities of 92.8% and 96.6%, as shown in Table 2. The difference in density of the 40 and 80 MPa samples can be explained by the shrinkage of the samples, as shown in Fig. 4. The densification of HAp starts early at 760°C for the sample under a pressure of 80 MPa compared to that under 40 MPa, which begins at 800°C. Furthermore, the densification of the HAp sample at 80 MPa stops at 967°C, before the soaking time at 1000°C. In contrast, the 40 MPa sample shows that the densification stops at the beginning of the soaking time at 1000°C. These data elucidate the effect of pressure is imperative to obtain a higher density of HAp.
The effects of pressure on the grain size are shown in the SEM images in Figs. 3(d) and (e). Indeed, a higher applied pressure i.e., 80 MPa, yields a smaller CHAp grain size. Table 2 presents the average grain size of 448 ± 96 and 283 ± 59 nm for sintered CHAp under the applied pressures of 40 and 80 MPa, respectively. The suppression of grain growth in Fig. 3(e) is likely due to the higher pressure, i.e., 80 MPa, which promotes densification while suppressing grain growth. Nevertheless, the hardness value of the sintered CHAp under different applied pressures does not differ (6.19 and 6.40 GPa for applied pressures of 40 and 80 MPa, respectively). The higher hardness value of CHAp under the 80 MPa applied pressure is likely due to the finer grain size than that of its counterpart. Table 2 reveals that under higher applied pressure, the indentation fracture toughness of CHAp is lower. In this regard, this result is in agreement with Furushima et al.,28) who reported a lower fracture toughness when the hardness of WC-FeAl increased. Launey and Ritchie29) explained that materials with small-sized grains inhibit crack propagation, which lowers the toughness.
4. ConclusionsHAps were successfully synthesized from natural resources, i.e., crab shells, using spark plasma sintering at 1000°C for 10 min under applied pressures of 40 and 80 MPa in a vacuum condition. The calcined crab-shell powder had an average particle size of 105 ± 28 nm, which is a typical result of the wet-chemistry method. Contrasting results were obtained for the calcined fish-scale powder, where large particle size and secondary phases of β-TCP were observed. Despite the higher temperature and longer sintering time (1200°C for 5 h), densification of CHAp pellets did not occur during pressureless sintering. In contrast, dense CHAp pellets were obtained with spark plasma sintering at 1000°C for 10 min. Moreover, a fine-grained microstructure was observed for spark plasma sintered CHAp under the applied pressures of 40 and 80 MPa, despite the lack of obvious difference in hardness. In conclusion, crab shell is an alternative to the existing HAp precursors, and further investigation is needed.
AcknowledgementsThe authors would like to acknowledge Mr. Iqbal and Mrs. Sapta from Kelola Mina Laut Company for providing the fishery waste materials used in this study.
Fig. 1XRD of calcined powder of CHAp at (a) 600°C and (b) 800°C; and FHAp at (c) 600°C and (d) 800°C. The calcination time for both powders was 5 h. 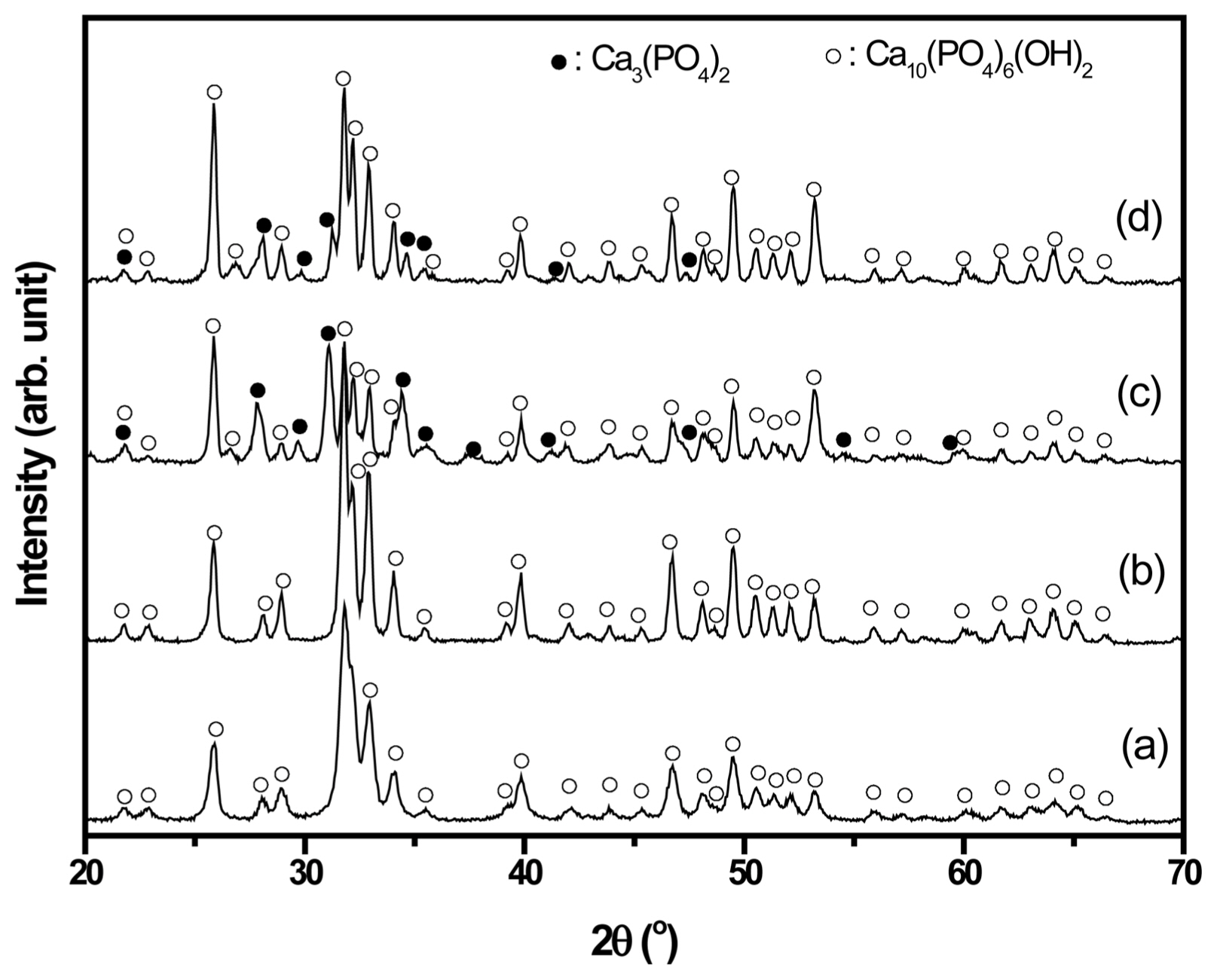
Fig. 2SEM images of calcined powder at 800°C for 5 h (a) FHAp and (b) CHAp. Two different magnifications were used for convenience. 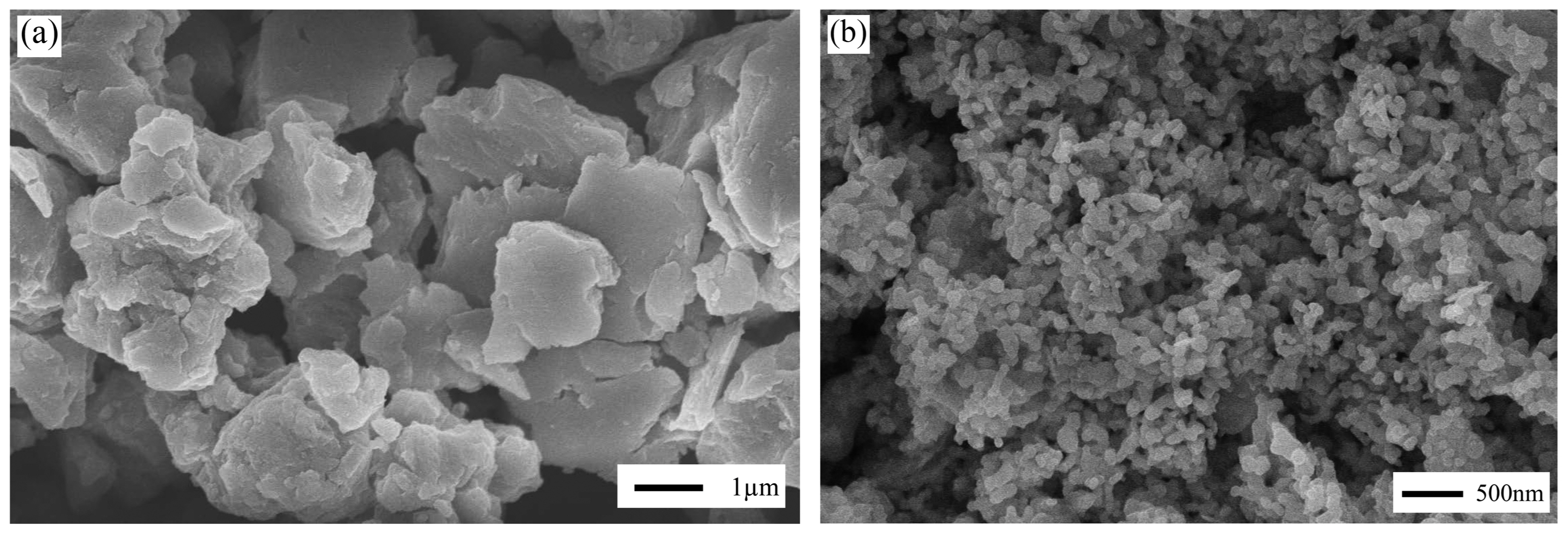
Fig. 3SEM of sintered CHAp by pressureless sintering for 5 h at (a) 1000°C, (b) 1100°C, and (c) 1200°C; and spark plasma sintering for 10 min at 1000°C in a vacuum under an applied pressure of (d) 40 MPa and (e) 80 MPa. 
REFERENCES1. Y. Wibisono, Biomaterial dan Bioproduk; pp. 24, UB Press, Malang, 2017.
2. M. Markovic, B. Fowler, and M. Tung, “Preparation and Comprehensive Characterization of a Calcium Hydroxyapatite Reference Material,” J Res Natl Inst Stand Technol, 109 [6] 553-68 (2004).
3. S. Wu, J. Wang, L. Zou, L. Jin, Z. Wang, and Y. Li, “A Three-Dimensional Hydroxyapatite/Polyacrylonitrile Wcomposite scaffold designed for bone tissue engineering,” RSC Adv, 8 [4] 1730-36 (2018).
4. J. Sun, and L. Wu, “Polyether Sulfone/Hydroxyapatite Mixed Matrix Membranes for Protein Purification,” Appl Surf Sci, 308 155-60 (2014).
5. Y. Wibisono, WA. Nugroho, and T-W. Chung, “Dry Degumming of Corn-Oil for Biodiesel Using a Tubular Ceramic Membrane,” Procedia Chem, 9 210-19 (2014).
6. HS. Liu, TS. Chin, LS. Lai, SY. Chiu, KH. Chung, CS. Chang, and MT. Lui, “Hydroxyapatite Synthesized by a Simplified Hydrothermal Method,” Ceram Int, 23 [1] 19-25 (1997).
7. MC. Barbosa, NR. Messmer, TR. Brazil, FR. Marciano, and AO. Lobo, “The Effect of Ultrasonic Irradiation on the Crystallinity of Nano-Hydroxyapatite Produced via the Wet Chemical Method,” Mater Sci Eng C, 33 [5] 2620-25 (2013).
8. S. Santhosh, and S. Balasivanandha Prabu, “Thermal Stability of Nano Hydroxyapatite Synthesized from Sea Shells through Wet Chemical Synthesis,” Mater Lett, 97 121-24 (2013).
9. MR. Saeri, A. Afshar, M. Ghorbani, N. Ehsani, and CC. Sorrell, “The Wet Precipitation Process of Hydroxyapatite,” Mater Lett, 57 [24-25] 4064-69 (2003).
10. M. Sivakumar, TS. Sampath Kumar, KL. Shantha, and K. Panduranga Rao, “Development of Hydroxyapatite Derived from Indian Coral,” Biomaterials, 17 [17] 1709-14 (1996).
11. D. Ulfyana, F. Anugroho, SH. Sumarlan, WA. Nugroho, and Y. Wibisono, “Bioceramics Synthesis of Hydroxyapatite from Red Snapper Fish Scales Biowaste Using Wet Chemical Precipitation Route,” IOP Conf Ser Earth Environ Sci, 131 012038(2018).
12. Y. Xu, D. Wang, L. Yang, and H. Tang, “Hydrothermal Conversion of Coral into Hydroxyapatite,” Mater Charact, 47 [2] 83-7 (2001).
13. S. Kongsri, K. Janpradit, K. Buapa, S. Techawongstien, and S. Chanthai, “Nanocrystalline Hydroxyapatite from Fish Scale Waste: Preparation, Characterization and Application for Selenium Adsorption in Aqueous Solution,” Chem Eng J, 215-216 522-32 (2013).
14. A. Shavandi, AEDA. Bekhit, A. Ali, and Z. Sun, “Synthesis of Nano-Hydroxyapatite (nHA) from Waste Mussel Shells Using a Rapid Microwave Method,” Mater Chem Phys, 149 607-16 (2015).
15. C. Piccirillo, RC. Pullar, DM. Tobaldi, PML. Castro, and MME. Pintado, “Hydroxyapatite and Chloroapatite Derived from Sardine by-products,” Ceram Int, 40 [8] 13231-40 (2014).
16. S. Mondal, S. Mahata, S. Kundu, and B. Mondal, “Processing of Natural Resourced Hydroxyapatite Ceramics from Fish Scale,” Adv Appl Ceram, 109 [4] 234(2010).
17. A. Prasad, B. Devendar, MR. Sankar, and PS. Robi, “Micro-Scratch Based Tribological Characterization of Hydroxyapatite (HAp) Fabricated through Fish Scales,” Mater Today Proc, 2 [4-5] 1216-24 (2015).
18. YC. Huang, PC. Hsiao, and HJ. Chai, “Hydroxyapatite Extracted from Fish Scale: Effects on MG63 Osteoblast-like Cells,” Ceram Int, 37 [6] 1825-31 (2011).
19. M. Boutinguiza, J. Pou, R. Comesaña, F. Lusquiños, A. De Carlos, and B. León, “Biological Hydroxyapatite Obtained from Fish Bones,” Mater Sci Eng C, 32 [3] 478-86 (2012).
20. A. Noviyanto, and D-H. Yoon, “Metal Oxide Additives for the Sintering of Silicon Carbide: Reactivity and Densification,” Curr Appl Phys, 13 [1] 287-92 (2013).
21. A. Noviyanto, YH. Han, and DH. Yoon, “Characteristics of SiCf/SiC Hybrid Composites Fabricated by Hot Pressing and Spark Plasma Sintering,” Adv Appl Ceram, 110 [7] 375-81 (2011).
22. YW. Gu, NH. Loh, KA. Khor, SB. Tor, and P. Cheang, “Spark Plasma Sintering of Hydroxyapatite Powders,” Biomaterials, 23 [1] 37-43 (2002).
23. Y. Watanabe, T. Ikoma, A. Monkawa, Y. Suetsugu, H. Yamada, J. Tanaka, and Y. Moriyoshi, “Fabrication of Transparent Hydroxyapatite Sintered Body with High Crystal Orientation by Pulse Electric Current Sintering,” J Am Ceram Soc, 88 [1] 243-45 (2005).
24. M. Eriksson, Y. Liu, J. Hu, L. Gao, M. Nygren, and Z. Shen, “Transparent Hydroxyapatite Ceramics with Nanograin Structure Prepared by High Pressure Spark Plasma Sintering at the Minimized Sintering Temperature,” J Eur Ceram Soc, 31 [9] 1533-40 (2011).
25. BN. Kim, E. Prajatelistia, YH. Han, HW. Son, Y. Sakka, and S. Kim, “Transparent Hydroxyapatite Ceramics Consolidated by Spark Plasma Sintering,” Scr Mater, 69 [5] 366-69 (2013).
26. AA. Gandhi, RD. Gunning, KM. Ryan, and SAM. Tofail, “The Role of Texturing and Densification on Optical Transmittance of Hydroxyapatite Ceramics,” J Am Ceram Soc, 93 [11] 3773-77 (2010).
|
|
||||||||||||||||||||||||||||||||||||||||||