1. Introduction
Digital ink-jet printing systems using nano-ceramic ink that stably develops color at high temperature have recently accomplished considerable technological advancements.1-2) The ceramic inks used in the digital ink-jet printing technology generally require four primary colors, cyan, magenta, yellow, and black (CMYK), to realize full-color images.3) In addition, nano-ceramic inks for inkjet printing, in order to be well jetted from a piezoelectric printer head, should have dispersion stability as well as an appropriate range of viscosity (4 to 40 mPa·s) and surface tension (20 to 45 mN/m).3-8)
Ceramic inks currently used for digital printing are prepared using organic solvents that are relatively good at securing the dispersion stability of hydrophobic ceramic pigments. However, due to regulations on the volatile organic compounds (VOCs) contained in organic solvents and the life-shortening of inkjet heads by organic solvents, there is a growing interest in environment-friendly inkjet printing materials employing water-based ceramic inks.9-10)
In the present study, a nano-sized CoAl2O4 ceramic pigment was used to prepare a water-based environment-friendly ceramic ink that has dispersion stability and shows good jetting characteristics when applied to an inkjet printing process. The drop formation behavior of the ceramic ink was analyzed depending on the ceramic ink properties required by inkjet printing. In addition, the printing characteristics of the prepared ceramic ink were investigated on ceramic tiles.
2. Experimental Procedure
The properties of a blue ceramic pigment (CoAl2O4, HANIL) applied to the ceramic inkjet printing process were evaluated by analyzing the crystal structure with an X-ray diffractometer (XRD, Rigaku, D/MAX2500VL/PC) and the particle shape and size through field emission scanning electron microscopy (FE-SEM, Jeol, JSM-6390). In addition, the BET (Brunauer-Emmett-Teller) surface area was analyzed using a BET surface measurement instrument (BET, TRISTA II 3200, Micromeritics, USA).
The dispersion of the ceramic pigment was tested using Na-Octanoate as an anionic surfactant and polyacrylic acid (Na-PAA, MW. 15000 g/mol) containing Na+ salt as an anionic polymer electrolyte. Each of the dispersants was added at a concentration in a range of 0 to 2.0 mg/m2 with reference to the surface area of the ceramic pigment; the resulting solution was stirred for 2 h to reach chemical equilibrium. Then, the CoAl2O4 pigment was added to the resulting solution. The ceramic ink containing the ceramic pigment at 35 wt% was milled for 72 h using a zirconia medium. The dispersant showing the best pigment dispersion as well as its mixing ratio was determined by analyzing the flowability and the sedimentation behavior.
In addition, the applicability to the inkjet printing process was analyzed by varying the surface tension and the viscosity of the prepared ceramic ink by respectively using a polysiloxane-based additive (BYK-28, BYK) and poly ethylene oxide (PEO, MW. 100,000, 200.000, 400,000 g/mol). The viscosity of the ceramic ink was measured using a rotational rheometer (HAAKE MARS III, Thermo Fisher Scientific Inc.); the surface tension was measured using a surface tension analyzer (DST-60, Surface Electro Optics Co.). A drop watcher (STI Co.) was also used to analyze the drop formation behavior in inkjet printing depending on the properties of the ceramic ink.
3. Results and Discussion
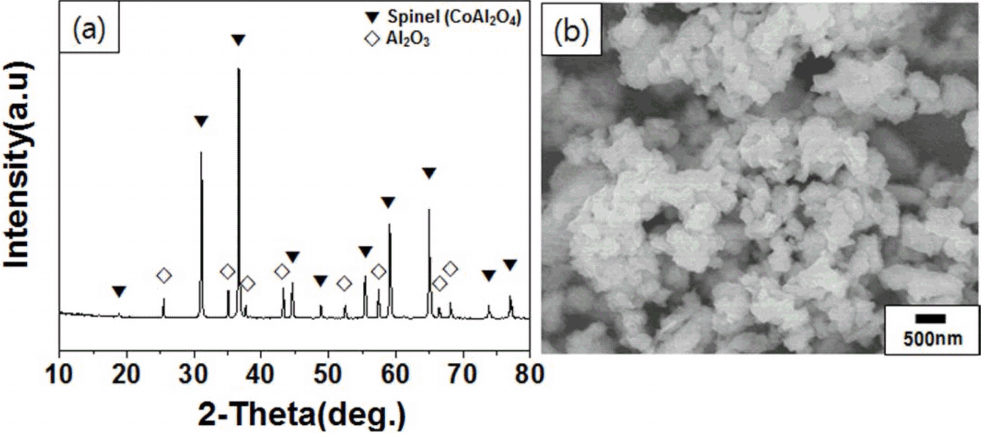
Figure 1 shows the crystal structure and the microstructure of the CoAl2O4 ceramic pigment. The XRD result shown in Fig. 1(a) indicates that the crystal structure of the ceramic pigment included a spinel face corresponding to CoAl2O4, represented by the main peak, and the corundum face corresponding to Al2O3. The result of the pigment particle size and microstructure analysis, shown in the FE-SEM image in Fig. 1(b), indicates that the size of the primary particles of the pigment were about 200 to 300 nm. Almost no coagulation was found between the pigment particles, and most of the particles were angular. The density and the specific surface area of the CoAl2O4 pigment were 4.44 g/cm3 and 5.55 m2/g, respectively, as shown in Table 1.
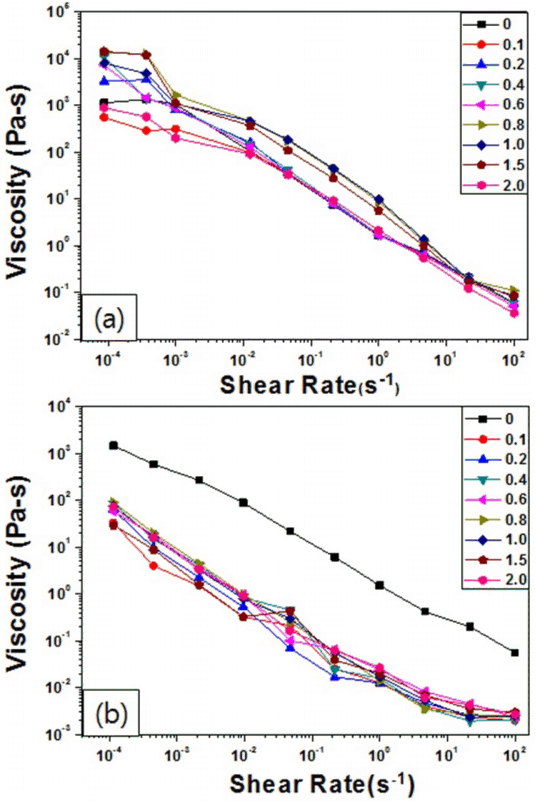
To secure the dispersion of the ceramic pigment, Na-Octanoate, an anionic surfactant, and Na-PAA, a polymer electrolyte dispersant, were respectively added to inks containing the CoAl2O4 ceramic pigment at 35 wt%; the viscosity of the ink depending on the shear rate was measured, with results shown in Fig. 2. Regardless of the type and the mixing ratio of the dispersants, non-Newtonian behavior was found in all the samples with shear-thinning. As the content of the added dispersant increased, the viscosity of the ceramic ink slightly decreased.
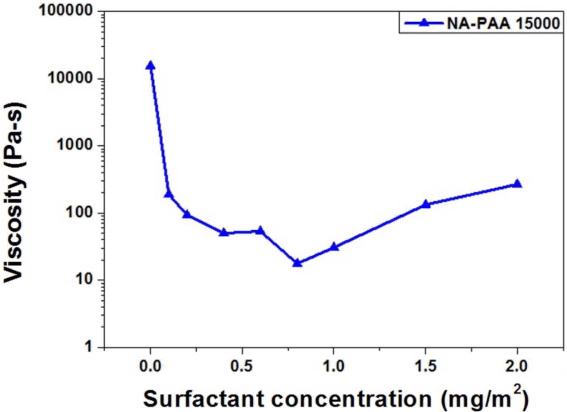
When Na-Octanoate was used as a dispersant, the viscosity of the ceramic ink did not significantly decrease as the content of the added dispersant increased, which may be because the anionic surfactant was not well adsorbed on the pigment particle surface or even because the adsorbed surfactant failed to show sufficient repulsive force between the pigment particles, having little effect on the dispersion. On the contrary, when Na-PAA was used as a dispersant, the viscosity significantly changed. Therefore, Na-PAA was selected as a more effective dispersant for the CoAl2O4 pigment. As the addition of the dispersant increased, the viscosity of the ceramic ink decreased, which indicates that Na-PAA was effectively adsorbed to the CoAl2O4 pigment surface and thus the dispersion was probably stabilized by the stabilization mechanisms of electrostatic stabilization and steric hindrance.11) Based on the results shown in Fig. 2(b), the apparent viscosity of the ceramic ink was measured depending on the content of the Na-PAA dispersant, as shown in Fig. 3. The dispersion of the ceramic pigment may have been most effective at the Na-PAA concentration of 0.8 mg/m2, with the lowest viscosity.12)
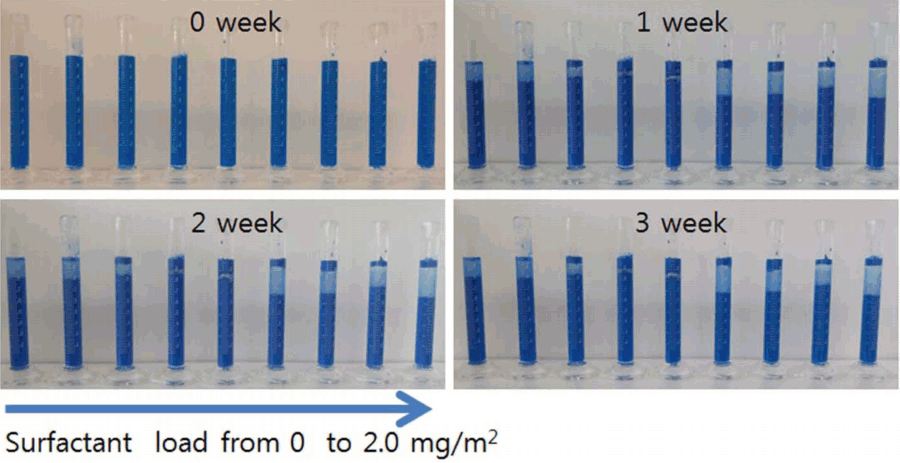
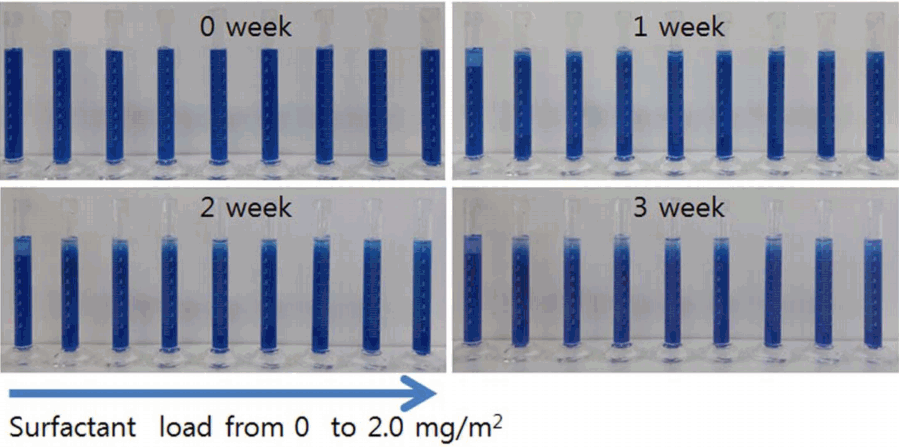
Figures 4 and 5 show the sedimentation behavior of the ceramic inks prepared using Na-Octanoate, which is an anionic surfactant, and Na-PAA, which is a polymer electrolyte dispersant, respectively. To prepare ceramic inks, each of the dispersants was added at a concentration in a range of 0 to 2.0 mg/m2 with reference to the surface area of the ceramic pigment. To analyze the dispersion stability, the sedimentation behavior was observed by keeping the ceramic ink in a measuring cylinder for two months. The flocculation of the ceramic pigment particles over time was investigated by measuring the volume of the bottom layer (sediment), middle layer (clarified), and top layer (float). Fig. 4 shows the sedimentation behavior of the ceramic ink prepared using Na-Octanoate, which is an anionic surfactant; it can be seen that the top layer was clarified from one day after the preparation. As time passed, the layers separated further. Such stratification may have been caused by the flocculation of the CoAl2O4 particles, which have high surface energy in the ceramic ink, over time. Fig. 5 shows the sedimentation behavior of the ceramic ink prepared using Na-PAA, which is an anionic polymer electrolyte. The sedimentation test showed that the sedimentation of the ceramic ink containing the anionic polymer electrolyte occurred slowly over three weeks. This shows that the repulsive force between the particles caused by the polymer adsorbed to the particles and the anions surrounding the polymer may have prevented flocculation and sedimentation of the particles.
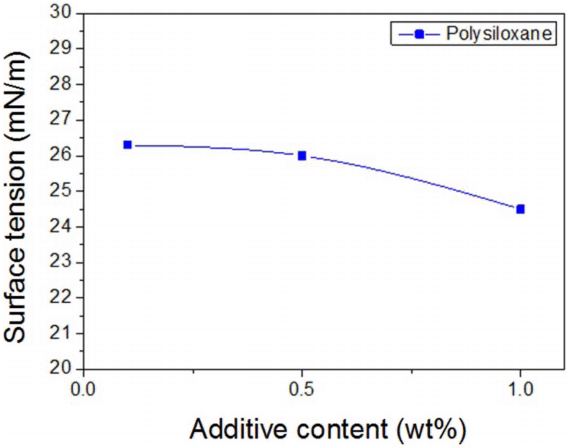
According to the optimal dispersion conditions mentioned above, the ceramic pigment was dispersed by adding Na-PAA at the concentration of 0.8 mg/m2; a polysiloxane-based additive to control the surface tension of the ceramic ink was added at 0.1 wt%, 0.5 wt%, and 1.0 wt% with reference to the pigment contained in the ink; then, the surface tension was measured, with results shown in Fig. 6. Control of the surface tension and the viscosity is a necessary factor for ceramic inks used for digital printing. In the preparation of ceramic inks, one of the important characteristics of a water-based system is that the high surface tension gives capillary force to the nozzle when jetting the ink. At the moment when a drop is separated from the nozzle, the surface area is instantaneously reduced to make the drop spherical, and the tail of the drop is quickly shortened. Therefore, in the smooth jetting of a water-based ceramic ink, it is critical to control the surface tension of the water-based ceramic ink within the appropriate range of 20 to 45 mN/m, as required in inkjet printing. The surface tension of the ink was 64 mN/m before the addition of polysiloxane, which is a surface tension modifier, and 26.3, 26.0, and 24.5 mN/m when the polysiloxane content was increased to 0.1 wt%, 0.5 wt%, and 1.0 wt%, respectively. As the mixing ratio of polysiloxane increased, the surface tension decreased. The surface tension appropriate for an inkjet printing process was achieved by adding just 0.1 wt% polysiloxane.
Figures 7 and 8 show the drop formation behavior of the ceramic ink as it depends on the surface tension and viscosity. All the ceramic inks were prepared by adding as a dispersant Na-PAA and polyethylene oxide (PEO, Mw. 100,000, 200,000, and 400,000 g/mol) of different molecular weights at 2%; then, the jetting characteristics were analyzed.
Figure 7 shows the drop formation of the ceramic ink with unadjusted surface tension. A convex meniscus was formed at 0 to 20 μs; the drop was elongated by the transferred pressure vibration at 40 to 60 μs; it then separated from the original drop at 80 μs. Due to the high surface tension, which tends to minimize the surface area of an elongated tail and drop, high tension acts inside the drop, separating individual drops and forming satellite drops. As the viscosity of the ink was increased by the addition of PEO to 4.35, 5.43, and 12.9 mPa·s, the distance between the original drop and the satellite drop was reduced.
Figure 8 shows the results obtained by lowering the surface tension of the water-based ceramic ink from 62 mN/m to 32.5 mN/m by adding polysiloxane 0.1%. Analysis of the jetting behavior showed that a meniscus was formed at 0 - 20 μs; the drop was elongated at 40 - 60 μs; then, the drop separated as a single independent drop at 80 μs. When the surface tension was controlled, stable jetting was found under all conditions.
To verify the applicability to inkjet printing, inkjet printing was performed on ceramic tiles using a ceramic ink with uncontrolled surface tension to form satellite drops in jetting (Fig. 7(c)); another ceramic ink with controlled surface tension showed stable jetting characteristics (Fig. 8(c)), as shown in Fig. 9. Fig. 9(a) shows that the ceramic ink having uncontrolled surface tension formed satellite drops, not a single drop, at the moment of ink jetting, resulting in printing at positions not in the desired pattern. This may cause low printing resolution. On the contrary, Fig. 9(b) shows that a single spherical drop formed when the viscosity and surface tension were controlled to be appropriate for the printing requirements. In addition, the results of printing with jetted ceramic ink showed that overlap between printed drops was minimized, and only the desired pattern was printed at the right position, as required by the user.
4. Conclusions
In the present study, a water-based ceramic ink was synthesized and applied to inkjet printing. The microstructure and the crystal structure of the selected ceramic pigment, CoAl2O4, were analyzed. For application to inkjet printing, the viscosity and the surface tension of the ceramic ink were properly controlled, and the applicability to inkjet printing and the drop formation behavior were analyzed. The CoAl2O4 pigment, having a particle size in a range of 200 to 300 nm, was dispersed using Na-PAA (Mw. 15,000 g/mol), which is an anionic polymer electrolyte. The best dispersion was found at the dispersant content of 0.8 mg/m2. In addition, the jetting characteristics of the ink were analyzed by optimizing the viscosity and the surface tension. For the printing of a clearer pattern, formation of satellite drops in the drop formation process was minimized in the inkjet printing process on ceramic tiles. This showed that the water-based ceramic ink prepared in the present study may be applied to digital printing processes.