1. Introduction
Dongwha or Jinsa is a red pigment inherent to our country with copper as the color forming composition. In 2005, National Museum of Korea has reviewed the problem of terms in the mixed use of Dongwha or Jinsa (copper-red pigment) and adopted “Dongwha” for use. Koryo Dongwha porcelain developed around the 12th century was the first case of color formation in red from the pigment of copper composition at high temperatures. Considering that such technology in China was started after Won Dynasty in the 14th century and became perfected during the early Ming Dynasty in the 15th century, the excellence in ceramic technology of our country can be supposed.1)
In the previous studies explaining color formation mechanisms for copper red glaze and copper ruby glass produced by using copper oxides, metallic Cu or copper oxide (I) particles formed in the reduction process were described as the cause for color formation.2-7) Such division in the opinions on the cause for color formation appears to be due to the differences in composition of the specimens analyzed and in heat treatment processes. While domestic investigators also considered metallic Cu as the main cause for color formation, BaO was used as one of the flux compositions in the case of Dongwha glaze used for analysis unlike the composition for Dongwha glaze in Koryo and Chosun era analyzed by Hwang Hyeonseong in 2008 in.8-10) Composition of the glaze can act as an element affecting not only glossiness, illuminance and texture but also color formation.11)
In the present study, the basic glaze was prepared based on composition of Dongwha porcelain in Chosun era, and the specimens of Dongwha glaze were prepared by variation in added amounts of copper oxide (II) and heat treatment atmospheres. To clarify the color formation mechanisms for Dongwha glaze, correlations with the colors were investigated by conducting spectroscopic, crystal phase, and microstructure. From here, attempts have been made to develop the technologies utilized for porcelain and inorganic pigment areas by defining the factors affecting color formation of our inherent Dongwha pigment, and by developing compositions and process technologies to realize stable colors.
2. Experimental Procedure
For glaze preparation, the basic glaze composition was determined by referring to the chemical composition analysis results of the excavated remains of Dongwha glaze. By unity molecular formula UMF method, the ratio between alkali and alkaline earth oxide was set to be 0.2 : 0.8, and the ratio between alumina and silica to be 0.46 : 3.50. For the alkaline earth oxide, CaO and MgO were used in a similar way to that for the Chosun Dongwha porcelain. The basic glaze was prepared by mixing Buyeo feldspar, sodium carbonate, limestone, magnesium carbonate, silica, kaolin, and alumina weighed according to the composition ratio with distilled water to the ratio of 65 wt.% for solid content. Copper oxide (II) of 0, 0.5, 1, 3, and 5 wt.%, respectively, was added to this mixture, and mixed by using ball mill for 24 h.
As the porcelain used for experiments, white porcelain capable of minimizing the effects due to impurities such as Fe2O3 and TiO2 was used. The bisque white porcelain specimens of 50 × 50 mm in size were sintered after plop glazing with 5 types of the glaze prepared above. Heat treatment atmosphere was controlled by using the mixed gas of air and liquefied petroleum gas, LPG. Air supply amount was fixed at 5 L/min, and the atmosphere was controlled by varying LPG supply to 0, 0.3, 0.5, and 0.7 L/min. For heat treatment, temperatures were raised at 3°C/min to 900°C where the reducing atmosphere was formed by supply of LPG, followed by being held at 1250°C for 1 h and subsequent natural cooling.
By using the spectrophotometer (CM-700D, Konica Minolta, Japan), the glaze surface was subjected to colorimetry for 5 times and average values of CIELAB and Munsell Color values were calculated by employing SpectraMagicTM NX Cm-S100W program. Absorbance characteristics for Dongwha glaze were analyzed by using UV-Vis-NIR spectroscopy (UV-Vis-NIR spectroscopy, Jasco, V-770, Japan). Crystal phase analysis in the glaze was conducted by using X-Ray diffractometer (Dmax-2500, Rigaku, Japan). Cross sectional microstructures of specimens were comparatively analyzed by using scanning electron microscope (JSM-6707M, Jeol, Japan), with EDS Mapping analysis being carried out. Cross sections of the glaze were observed by using transmission electron microscope (Tecnai F30, FEI, New Zealand).
3. Results
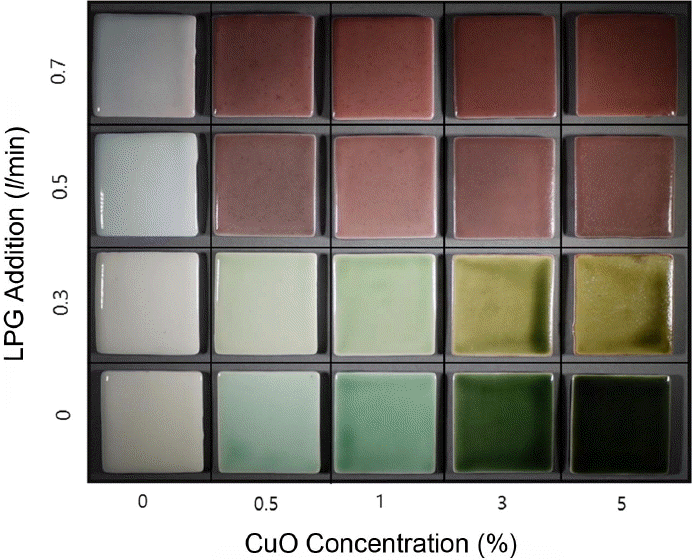
Specimens prepared by varying the copper oxide content and the heat treat were summarized in Fig. 1. Under the condition of 0 L/min LPG, Dongwha glaze exhibited color formation in G-series, while it showed color formation in GY-series under the condition of 0.3 L/min LPG. Under the conditions of 0.5 and 0.7 L/min LPG, it showed color formation in R-series. Based on the color formation characteristics of the specimens, heat treatment can be presumed to have been realized in the oxidative atmosphere at 0 and 0.3 L/min LPG, while it can be presumed to have been realized in reductive atmosphere in the furnace at 0.5 and 0.7 L/min. Although smooth glaze surfaces and stable gloss were commonly observed up to 1 wt.% content, irrespective of heat treatment atmospheres, rough glaze surfaces due to bubbles could be affirmed with the copper oxide content as increased to 3 and 5 wt.%. Also, degradation in the gloss was observed due to dull color formation of glaze and bubbles. Thus, the copper oxide (II) may be inferred to act as the cause for production of the bubbles.
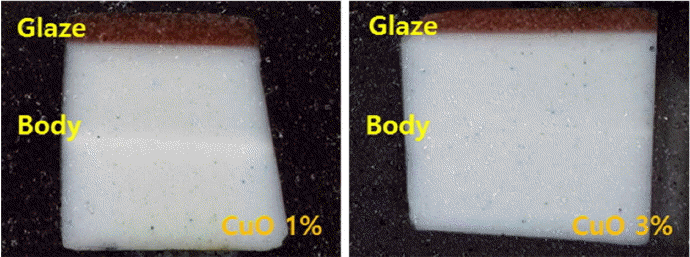
To check whether the color formation of Dongwha glaze specimens is a phenomenon occurring in a part of the glaze layer, the specimens prepared in the atmosphere of 0.5 L/min. LPG were cut for observation of the cross sections of the glaze layer. As shown in Fig. 2, the color formation in glaze was affirmed to be the phenomenon occurring homogeneously throughout rather than in a limited part.
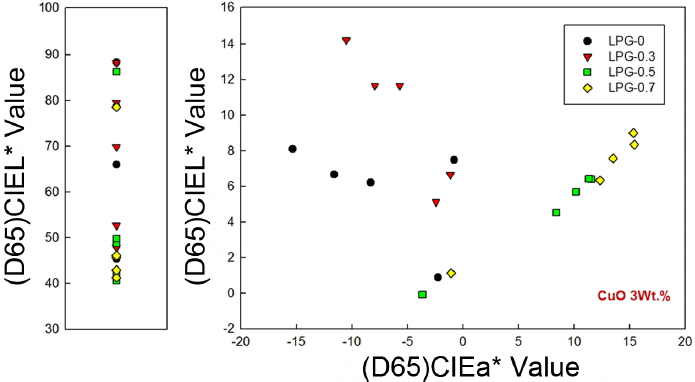
Distributions of CIELAB in Dongwha glaze specimens are shown in Fig. 3 as a function of the copper oxide (II) content per heat treatment atmosphere. Under the conditions of 0 and 0.3 L/min LPG, it was affirmed that CIEa* value became a ‘−’ value and CIEb* value a ‘+’ value as the copper oxide (II) content were increased so as to be distributed in Green series and Green-Yellow series, respectively. On the other hand, under the conditions of 0.5 and 0.7 L/min LPG, CIEa*b* distribution became a ‘+’ value in direct proportion as the copper oxide (II) content were increased, showing progression to Red series as the reducing atmosphere became intensified. Depending on the oxidation-reduction atmosphere, a* values for the specimens were affirmed to be increased in the opposite direction and b values increased in the same (+) direction as the copper oxide content were increased.
For the specimens with 3 wt.% of copper oxide (II) heat treated in different atmospheres, absorbance characteristics were analyzed by using UV-Vis-NIR spectroscopy (Fig. 4). The specimens heat treated at 0, 0.3, 0.5, 0.7 L/min LPG showed different results. The specimens heat treated at 0 and 0.3 L/min LPG showed a broad peak near the wavelength of 750 nm, while those heat treated at 0.5 and 0.7 L/min can be seen to show a high absorbance up to 560 nm followed by a rapid drop.
No narrow peak near 570 nm representing metallic nanocluster of Cu reported in the literatures were observed in all specimens.5,12) Also, the broad peak near 430 nm associated with isolated Cu atoms did not appear. Existence of metallic copper atoms and nanoclusters which were reported as the major cause for color formation in Dongwha glaze could not be affirmed through UV-Vis-NIR spectroscopy analysis. In the case of oxidized specimens, all broad peaks were observed near 750 nm due to Cu2+. Intensities of the peak can be seen to be increased as the oxidative atmosphere became more intensified. Chromaticity of the specimens sintered in oxidative atmosphere can be seen to have green color due to the presence of Cu2+.
Crystal phases of the glaze layer were analyzed by using XRD. As shown in Fig. 5, broad peaks and quartz peaks were observed for 2θ = 20 - 30° showing amorphous phases at 0 L/min LPG, while the peaks of quartz and metal Cu appeared at 0.3 L/min. Under the conditions of 0.5 and 0.7 L/min LPG, all peaks corresponding to Cu, CuO and Cu2O as well as quartz could be seen to appear. As the reductive atmosphere became intensified, the intensity of metal Cu peaks can be seen to become relatively stronger.
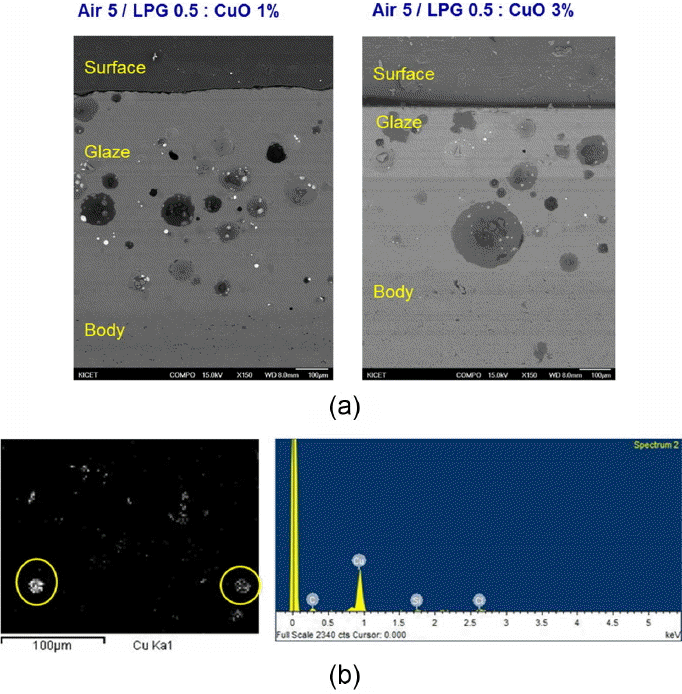
The observation results for microstructure of glaze layer using SEM are summarized in Fig. 6. In the case of specimens heat treated at 0 and 0.3 L/min for the added amounts of LPG, no unique crystal formation, etc. were observed. In the specimens heat treated at 0.5, and 0.7 L/min LPG, Cu particles of 10 microns in size were observed around bubbles in the glaze layer. The distribution and the size of Cu particles showed no correlation with the heat treatment conditions and the CuO content. Nano-sized Cu crystals discussed in many articles were not observed in any specimen in the observations using TEM (Fig. 7).
4. Discussion
CuO employed as the Cu source for Dongwha glaze is known to exist in diversified forms depending on the heat treatment temperatures and atmospheres.2,13,14) While copper oxide (II) is dissolved to form Cu2+ in oxidative atmospheres, the specimens heat treated at 0 and 0.3 L/min LPG appear to exhibit green color. This is supported by the broad peak near the wavelength of 750 nm observed in UV-Vis-NIR spectroscopy analysis results. According to the XRD analysis results, formation of some metal Cu could be affirmed to occur in a weak oxidative atmosphere (0.3 L/min LPG). This suggests that Cu2+ dissolved in high temperatures has been reduced by combination with electrons produced in a glass structure or impurities.15)
Copper oxide (II) reacts with CO gas produced by combustion of LPG gas for reduction to Cu2O or Cu.16-19) According to the existing studies, CuO may be reduced in the order of Cu2O-Cu, or directly to Cu, while the process of direct reduction to Cu is known to be preferred at relatively low temperatures. The rates of reduction to Cu are affected by CO gas concentration and temperature, and the reduction rates are expected to be increased in the present experiment when the flow rate of LPG gas are increased. Therefore, when the flow rate of LPG are increased, the reduction rates of CuO present in the glaze are increased so that the peak intensity of metal Cu also appears to be increased in the XRD analysis results.
Plewa affirmed that reduction to Cu occurred within 1 h when a minor amount of CuO was exposed at 200°C to the mixed gas of CO-CO2 similar to the combustion gas of LPG.17) Considering such observation, most of copper oxide (II) added to the glaze up to the maximum 5% appears to be reduced to metal Cu state under the assumption that sufficient CO gas was produced in the heat treatment process (of being held at 1250°C for 1 h after temperature rise at 3°C/min) applied in the present experiment. However, in the case of porcelain glaze, the liquid phase material formed by melting of the raw material as the temperature was raised comes to play a role of limiting the contact of CuO with CO gas. The glaze employed in the experiment was melted to form a liquid phase near 900°C where LPG gas began to be fed. The reduction process of CuO-Cu begins as CO gas was adsorbed to the Cu sites of CuO, and the liquid-phase glaze formed around CuO powder would limit the adsorption of CO gas, because of which it is expected to act as a factor slowing down the reduction rates as well as the reduction intensity. Therefore, the reduction intensity of CuO is considered to be affected by both CO gas adsorption and formation rate of liquid phase.
Copper oxide (II) reacts with CO gas to form Cu nuclei in the reductive atmosphere, which are known to form Cu nanocluster capable of acting as the cause for red color after growth.3) Further growth is known to cause the formation of metal Cu crystals having black color. Although all metal Cu peaks were affirmed according to the XRD analysis for the specimens produced at 0.5 and 0.7 L/min LPG, no peaks showing Cu nanocluster were observed in the analysis results of UV-Vis-NIR spectroscopy. Also, no Cu nanoclusters were visible in the microstructure observations using SEM and TEM. On the other hand, multitudes of metal Cu globules were affirmed to exist around bubbles within the glaze layer according to the SEM analysis results.
No observation of Cu nanoclusters within the glaze layer is associated with the multitudes of Cu metal globules which have grown to a size larger than 10 microns. The metal Cu formed by reaction with CO gas is expected to form aggregation to lower the system energy and to grow causing precipitation around bubbles during the cooling process. When the aggregation and the precipitation occur, Cu concentration within the glaze layer is relatively lowered, extending the distance for Cu nuclei to move for formation of clusters. For such reason, it appears that no clusters of a few ten nm in size were observed whereas metal Cu crystals of micron sizes were observed in the microstructure analysis. Based on the analysis results of UV-Vis-NIR spectroscopy, metal Cu aggregation of smaller than 10 nm is considered to have been formed.20,21)
According to the XRD analysis results, crystal phases of CuO and Cu2O were observed in the specimens sintered in the reductive atmosphere. However, crystal shapes corresponding to the same were not observed in the microstructure analysis for the glaze layer. This seems to be originated from the oxidation reaction in cooling process. For the metal Cu particles formed near the glaze surface, the chemical state is known to be affected by atmospheres during the cooling process.2,22) In the present experiment, feeding of LPG gas controlling the reductive atmosphere was stopped after being held at the maximum temperature for 1 h, and the cooling process was conducted within the furnace which was not completely sealed. Thus, the metal Cu particles formed in the surface layer of specimen during this process appear to be capable of forming Cu2O and CuO by oxidation.
Copper oxide (II) produces O2 or CO2 gas in both oxidative and reductive atmospheres. Because of this, the glaze surface appears to be roughened due to bubbles when the added amount of copper oxide is more than 3%. Therefore, the use of less than 1% of copper oxide is considered desirable to prepare Dongwha glaze with smooth gloss.
5. Conclusions
Specimens of Dongwha glaze were prepared by variation in the copper oxide (II) content and the heat treatment atmospheres. Under the oxidative atmosphere conditions of 0 and 0.3 L/min LPG, CIEa* value became a ‘−’ value and CIEb* value a ‘+’ value for color formation in Green series and Green-Yellow series as the added amounts of copper oxide (II) were increased. On the other hand, under the reductive atmosphere conditions of 0.5 and 0.7 L/min LPG, CIEa*b* distribution became a ‘+’ value in direct proportion for color formation in Red series.
The specimens sintered in the oxidative atmosphere could be seen to have green color due to Cu2+ formed by dissolution of CuO. For the specimens sintered in the reductive atmosphere, Cu nuclei aggregation and existence of metal Cu globules of ~10 microns in size were affirmed in the glaze layer, while Cu2O and CuO were formed in the surface layer due to the oxidation during cooling process. Although the reduction specimens showing color formation in Red series of low chroma and dull tone appear to be affected by the combined effects of Cu nuclei aggregation, metal Cu globule and Cu2O, no quantitative analysis of the same was conducted in the present study.
Since copper oxide (II) produces gas in both oxidative and reductive atmospheres, less than 1% of copper oxide should be used for Dongwha glaze to allow preparation of smooth and glossy Dongwha glaze.