1. Introduction
Li-ion batteries are mainly used to power portable electronics including cell phones, laptop computers, and electric vehicles. However, the Li-ion battery technology has several limitations including safety issues, cycle life, and high cost. Furthermore, lithium ion batteries are now being utilized on a large scale. In this light, Na-ion batteries have become an important alternative because of their high energy density, easy availability, and the environmental benignity of sodium.1-4)
However, it is necessary to develop high-performance sodium battery cathodes by identifying new intercalation hosts and/or performing structural modifications of existing materials using various synthetic strategies. The choice of phosphate-based Na-insertion hosts is due to the strong covalent (PO4)3− units that provide structural stability even at high charge states and address thermal safety concerns, in contrast with commercial Li-insertion oxide hosts such as LiCoO2.5) Oxide materials may undergo oxygen evolution at high states of charge and elevated temperatures, potentially leading to explosions.6) Phosphate based materials, such as NaVPO4F,7) NaMPO4,8,9) Na1.5VOPO4F0.5 10) and Na2FePO4F,11,12) have been studied as active materials. Among them, only a few iron-based polyanionic compounds have been characterized and tested for sodium ion batteries. Na2FeP2O7 shows a theoretical capacity of 97 mAh/g with promising electrochemical properties involving Fe3+/Fe2+ redox activity at 3.0 V. Moreover, its 3D framework provides facile ionic conduction through its open channels, which are able to accommodate several sodium sites.13,14) Most of the studies reported on P2O7-based materials synthesized by conventional high temperature solid-state reactions.7,15) However, conventional solid-state reactions require high sintering temperature and long sintering time inducing unwanted particle growth which leads to poor electrochemical properties. A cost-effective and simple strategy to synthesize Na2FeP2O7 is hence highly desired.
In this work, we synthesized triclinic Na2FeP2O7 powders with high crystallinity by a one-step pyro-synthetic strategy using a polyol medium at a low temperature within a few minutes of reaction time in open-air condition. The polyol medium acts as a primary fuel to induce flame that can provide high thermal energy and carbon source.16) We demonstrated that by using polyol-assisted pyro-synthesis, a pure phase Na2FeP2O7 sample can be successfully obtained. More importantly, the sample showed good cycling stability when tested for a Na-ion battery.
2. Experimental Procedure
Na2FeP2O7 powders were prepared by a polyol-assisted pyro-synthetic method using sodium acetate (CH3COONa, 99.0%. Sigma Aldrich), iron acetate (Fe (CO2CH3)2, 99.99%, Sigma Aldrich), and phosphoric acid (H3PO4, ≥ 85%, Daejung). The precursors were dissolved in a beaker containing 80 mL of tetraethylene glycol (99.5%, Daejung) and the solution was mixed. After stirring for 5 h, phosphoric acid was poured and the solution was stirred for 24 h at room temperature. The overall molar ratio was 2 : 1 : 2 (Na : Fe : P). After subsequently obtaining a homogenous solution, 50 mL of inflammable liquid thinner was added to the solution, which was then stirred for an hour. The final solution was uniformly poured onto a hot-plate maintained at 470°C. The solution was ignited using a torch to induce incomplete combustion and the flames continued for a few minutes. After being self-extinguished, Na2FeP2O7 powders were obtained. Finally, the powders were heat-treated at 650°C for 6 h in Ar to remove the remaining organic compounds.
Powder X-ray diffraction (XRD) data of the samples were recorded using a Shimadzu X-ray diffractometer with Ni-filtered Cu Kα radiation (λ = 1.5406 Å) operated at 40 kV and 30 mA within a scanning range angle from 10° to 80° (2θ). The particle morphologies and sizes were determined by FE-SEM images that were obtained using a Hitachi S-4700. The electrochemical properties of the Na2FeP2O7 samples were evaluated with sodium metal as the reference electrode using a Nagano 2004H battery tester system (Nagano Keiki Co., Ltd., Ohtaku, Tokyo, Japan). The fabricated cathode consisted of active materials with 30% conductive carbon (Ketjen black) and teflonized acethylene black (TAB). The mixture was pressed onto a stainless steel mesh and dried under a vacuum at 120°C for 12 h. A 2032 coin type cell consisting of the cathode and a sodium metal anode separated by glass fiber was fabricated in an Ar-filled glove box and aged for 12 h before the electrochemical measurements. The electrolyte employed was 1M NaClO4 in propylene carbonate (PC). The assembled cells were tested at 0.08 C in a voltage range from 2.0 to 4.0 V.
3. Results and Discussion
The XRD patterns of the as-prepared and annealed Na2FeP2O7 samples are shown in Fig. 1. The diffraction peaks of the as-prepared sample were broad, indicating an amorphous nature. In contrast, the diffraction patterns of the sample heated in an argon atmosphere was well indexed to the triclinic phase of Na2FeP2O7 without any impurities.17) However, according to the element analysis results, the amount of carbon from combustion synthesis of the heated Na2FeP2O7 is around 1.42%. No carbon phase is detected from the XRD, indicating that the residual carbon in Na2FeP2O7 is in an amorphous state.18-20) Therefore, the weight of the active materials in the samples was corrected during galvanostatic measurement. Interestingly, the three dimensional Na2FeP2O7 crystal structure provides large channels for facile Na+ ion diffusion. This three dimensional structure is formed by corner sharing FeO6 octahedra creating Fe2O11 dimers, and these dimers are further interconnected by both corner-sharing and edge-sharing with P2O7 pyrophosphate groups.21)
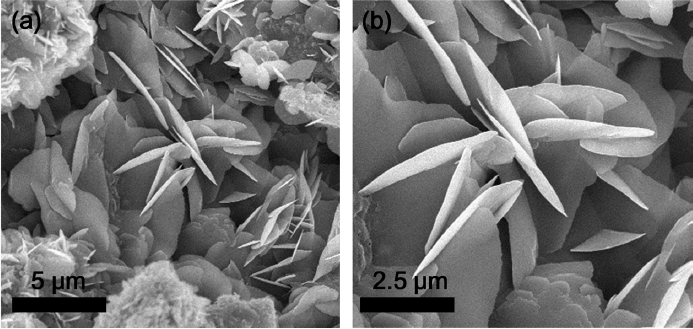
The morphology and the particle size of the samples were examined by FE-SEM, as shown in Fig. 2. The image of Na2FeP2O7 sample showed cross linked nano-plates where the average particle size was found to be around 2-5 μm and the particles were agglomerated. Nano-plate particles may facilitate passage of the electrolyte through each nanoplate, and thus more surface sites would be accessible for sodium ions to react with the Na2FeP2O7 sample. In addition, the cross linked structures enhance the electrode-electrolyte contact area and reduce the diffusion distance of the Na+ ions, and these factors play a key role in stable cyclability.22) It is also worth noting that high energy and short reaction time facilitate rapid nucleation and prohibit grain growth, leading to the formation of crystalline particles from the polyol-assisted pyro-synthesis. Further, the polyol medium, which acts as a conductive carbon source and limits the particle growth.16,23,24) During the fast combustion, the polyol can be pyrolyzed, which also results in carbonization of the polyol.16) The carbonized structures act as physical barriers to prevent particle growth and also form a thin conductive carbon layer coated on the Na2FeP2O7 particles. This improves the electron conductivity, thus resulting in good electrochemical performance.
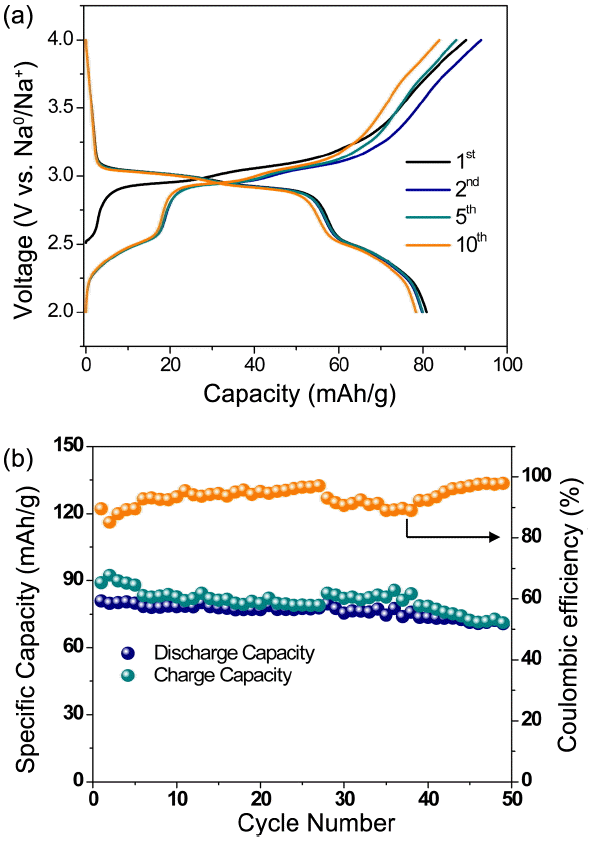
The electrochemical performance of the Na2FeP2O7 electrode vs. Na0/Na+ is presented in Fig. 3. The charge/discharge curves of the Na2FeP2O7 cycled between 2.0 and 4.0 V at 0.08C are shown in Fig. 3(a). Initial discharge capacity of 80.8 mA h/g was observed with a small plateau at 2.5 V and a large plateau at 3 V, which can be ascribed to structural rearrangement or Na-ion ordering during the electrochemical reaction.13)
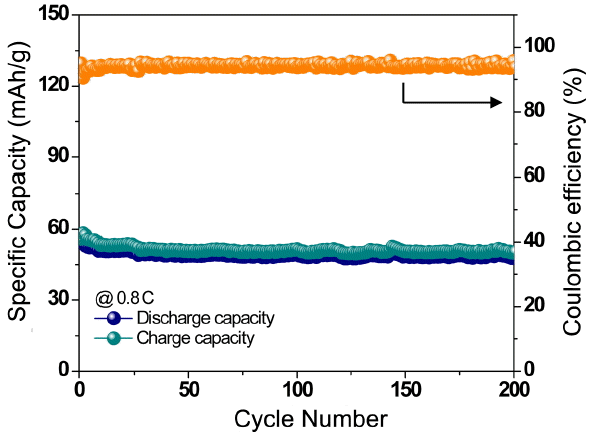
The cycle performance of the Na2FeP2O7 electrode is shown in Fig. 3(b). The discharge capacity showed stable performance with 90% of the initial capacity retained after 50 cycles at a 0.08 C rate for the electrode (73 mA h/g). Moreover, almost 100% Coulombic efficiencies are maintained even under prolonged cycling, as can be observed in Fig. 3(b). To further examine the rate performance, we investigated the specific capacity of the Na2FeP2O7 cathode under various current densities from 0.08 to 10.5 C in a potential range of 2.0 - 4.0 V by cycling it three times at each rate, and the results are shown in Fig. 4(a). The Na2FeP2O7 electrode exhibited discharge capacities of 46 and 16 mA h/g at 1.31 and 5.25 C, respectively. The capacity of the electrode recovered to 82 mA h/g at a rate of 0.08C, demonstrating the stability of the electrode. At a current density of 0.08 C, two plateaus can be observed in the discharge curve from Fig. 4(b). Further, at a higher current density, the plateaus become less clear, indicating larger polarization. To investigate long-term cycling, the Na2Fe P2O7 electrode was cycled at a rate of 0.8 C. The results in Fig. 5 showed good cycling stability performance of the electrode. The electrode registered first and 200th discharge capacities of 56 and 48 mA h/g, respectively. Further, as evidenced form Fig. 5, the Coulombic efficiencies are maintained at around 95% upon extended cycling. The excellent electrode properties are mainly attributed to the highly cross linked structure comprising nano-plate Na2FeP2O7 with carbon content.
4. Conclusions
In summary, carbon-coated Na2FeP2O7 cathode materials were successfully synthesized by a simple and low-cost pyro-synthesis method. The XRD patterns of the sample were well matched to triclinic Na2FeP2O7 without any impurity phase. The sample showed average particle size of about 2 - 5 μm with a cross linked nano-plate shape. The prepared Na2FeP2O7 cathode demonstrated initial discharge capacities of 80 mA h/g within a potential window of 2-4.0 V and showed good cyclability under long term cycling. The pyro-synthesis strategy offers opportunities to prepare carbon-coated cross linked nanoplate particles, which are not only well connected with the electrode and electrolyte and give short ion diffusion paths but also improve the electron conductivity, thus resulting in good electrochemical performance. In addition, this technique appears to be useful for synthesizing a variety of other materials suited for next generation Na-ion batteries.