Chang: Preparation of Anatase Particles through Electro-Dialysis of TiCl4 Aqueous Solution
Abstract
Anatase particles of titanium dioxide were prepared from TiCl4 aqueous solution by using an electro-dialysis [ED] process. For the preparation of an aqueous solution of TiCl4 precipitates, TiCl4 liquid frozen in ice was transferred to a neck flask and then hydrolyzed using deionized [DI] H2O. During the hydrolysis of the TiCl4 solution at 0°C, a slurry solution of TiOCl2 was obtained and the color changed from red to orange. The ED process was applied for the removal of chlorine content in the slurry solution. Two kinds of hydrolyzed slurry solution with lower [Ti4+] and higher [Ti4+] were sampled and the ED process was applied for the samples according to the removal time of [Cl−]. With de-chlorination, the solution status changed from sol to gel and the color quickly changed to blue. Finally, white crystalline powders were formed and the phase was confirmed by XRD to be anatase crystallites. The morphology of the hydrous titania particles in the solution was observed by FE-SEM. The hydrous titania particles were nano-crystalline, and easily coagulated with drying.
Key words: Titanium tetrachloride, Anatase, Electrodialysis, Nano-particles, Anion membrane
1. Introduction
Because it is nontoxic, easy to fabricate, inexpensive, and chemically stable, TiO 2 has been widely investigated and used in materials over the past several decades. Synthesis of highly crystalline titania nano-particles with controlled crystal structure, morphology, and size has been a very active field in materials chemistry. 1-4) In recent years, TiO 2-based nano-materials have attracted significant research attention due to their broad applications in the fields of water and air purification, H 2 production, and photovoltaic and photo-electrochemical cells. TiO 2 is a promising material for photo-electrochemical energy production and as such is an attractive material for solar energy conversion and as an effective catalyst for photo-oxidizing of a variety of hazardous organic chemicals in air and in water at room temperature. Among three polymorphs, anatase (tetragonal, metastable), rutile (tetragonal, stable), and brookite (orthorhombic, metastable), the metastable anatase-type TiO 2 has been known to exhibit the highest photocatalytic activities 1); this polymorph transforms into a thermodynamically stable rutile phase with heat treatment above 635°C, as was determined in a kinetic study on the transformation from anatase to rutile. 4) The phase transformation temperature of metastable inorganic materials is affected by their grain size, impurities, compositions, precursor materials, processing, and by the nature and amount of dopants. Recently, intensive attention has been devoted to wet chemical routes to obtain nanometer-sized particles of inorganic materials. Preparations of fine TiO 2 powders and their precursors have been examined using several techniques. 4-6)
In this study, an aqueous solution of TiOCl 2 was prepared by hydrolysis of TiCl 4 solution in ice water; then, an electrodialysis [ED] process 7-9) was applied to the solution. The application of an ED process in a TiOCl 2 solution was very effective for the preparation of TiO 2 nano-particles without chlorine residue. The particle morphology of the prepared TiO 2 nano-particles was analyzed using FE-SEM. The slurry solution of TiO 2 nano-particles was filtered using DI H 2O and, finally, ethanol solution. The dry sample was used for XRD and FE-SEM analysis.
2. Experimental Procedure
2.1. Preparation of TiOCl2 solution through hydrolysis
In the hydrolysis process, as shown in Fig. 1(a), TiCl 4 (99.0% purity, Sigma Aldrich) was carefully transferred to a neck flask under ice temperature and dissolved in deionized [DI] H 2O. The frozen TiCl 4 solution in a bottle was transferred to the reactor through a double tip cannula needle line under N 2 gas atmosphere. An inert atmosphere of pure N 2 gas was provided using a Schlenk line system. The TiCl 4 solution in the received bottle can be violently volatilized to HCl gas if the cover is opened in the air. In order to prevent a violent exothermic reaction with H 2O, the bottle was kept under ice temperature and N 2 atmosphere. We put a rubber septum cap into the bottle, which was kept under N 2 atmosphere in a glove box. The rubber capped bottle was frozen in the refrigerator. Just before the TiCl 4 transfer to the reactor, the frozen bottle was taken out and put in an ice water bath; this was similar to the procedure used with the Dewar flask model, 6) which is shown in Fig. 1(a).
Hydrolysis of TiCl4 solution
For the hydrolysis of the TiCl 4 solution, using a syringe needle, DI [deionized] water was slowly added dropwise under stirring through the flask septum. A liquid phase hydrolysis reaction of TiCl 4 was also carried out. 6) In Fig. 1(b), it can be seen that an orange color solution was obtained due to the formation of TiOCl 2. 6,10-13) The addition of DI water to TiCl 4 solution induces a strong exothermic hydrolysis reaction of TiCl 4, with the generation of HCl gas. Because of the highly exothermic reaction, the solution temperature quickly became hot. The overall process, such as the stirring speed and the addition of DI H 2O, was carefully controlled to maintain the reaction temperature in the salt-ice bath. TiCl 4 is known to react violently with water, releasing highly corrosive and toxic hydrogen chloride (HCl) gas. 6,13,14) The HCl gas pressure in the reactor was very strong, and so the septum rubber cap was tightly sealed to the flask neck with copper wire. It was also important to maintain appropriate N 2 gas pressure in the cannula line through the septum. In the reactor, N 2 gas has to be slowly injected in order to prevent the hydrolysis reaction. For the prevention of gas pub-up of the septum, the flask was frozen in a salt ice bath and the addition of H 2O was slowly controlled under stirring in an N 2 gas atmosphere. The transferred TiCl 4 solution was stirred for 20 min; then, DI H 2O was slowly injected into the reactor. The reaction with H 2O is very strong and exothermic. The reactor has to be strongly stirred and frozen using salt ice to prevent overpressure generation of HCl in the reactor. The starting precursor solution was TiCl 4 [Sigma Aldrich, 99.8%]. The stock solution of TiCl 4 was received in a bottle, which was frozen in salted ice water and transported to the flask reactor at 0°C. Pieces of block ice were added to the reactor to suppress fast hydrolysis when frozen water was slowly added to the flask reactor. The concentration of TiCl 4 was 2 mole/L; it was found that the stock solution could be kept for a year at room temperature without precipitation. During hydrolysis, the solution color was red and then changed to orange with the increase of H 2O addition. At room temperature, the orange color solution was transferred to the ED [ElectroDialysis] process reactor for dechlorination, as can be seen in Fig. 1(C). Anatase titania nano-crystallites were prepared using a low concentration of TiCl 4 in water, at a molar ratio of TiCl 4/H 2O = 1/15 and a high concentration of TiCl 4 (TiCl 4/H 2O = 1/10) in the temperature range of 40-60°C. Small, uniform, and yet size-tunable (5 - 10 nm) nano-crystalline TiO 2 (anatase) powders were prepared by electro-dialysis of TiOCl 2 in aqueous chloride solutions. 7,9)
The details of the experimental process are as follows:
septum rubber cap was attached to a TiCl4 precursor bottle in a glove box under N2 atmosphere using an ice bucket. Using a Schlenk line system, septum rubbers were attached to the necks of the reactor flask through vacuum and filling of N2 gas. The frozen TiCl4 bottle was moved to a salt-ice water bath and the TiCl4 bottle was connected to the reactor flask neck using a Cannula line. By controlling the N2 gas pressure in the Schlenk line system, it was possible to transfer the TiCl4 solution to the flask. Under N2 gas in the reactor, DI H2O was injected through another neck using a syringe. The reactor was rapidly stirred during the hydrolysis reaction. After the termination of hydrolysis, the reaction precipitates at room temperature were poured into the ED reactor.
During the hydrolysis reaction, [Ti4+] was controlled at 0.5M by the addition of iced H2O; solution was stirred for an hour. The precipitation reaction occurred at room temperature. After the termination of the precipitation reaction, the slurries of the precipitates were moved to the ED reactor in order to remove Cl− ions. After 10 minutes of ED process, the solution color changed from orange to blue and the solgel transition was simultaneously observed.
The DI water was purified by passing it through a PTFE filter (0.2 μm). After the de-chlorination, the precipitates were dried at 60°C for 12 h. In order to prevent the de-flocculation of the precipitates without Cl−, the precipitates were washed using DI H2O and ethanol.
2.2. ED reactor and Removal of Cl− in Ti-OH-Cl solution
At room temperature, the mixture solution of Ti-OH-Cl was transferred to the reaction chamber, which had a cathode, as shown in Fig. 1(C); the ED process was performed for the removal of Cl − ions, as has been previously reported. 7,8) With the progress of the ED reaction, it was possible to observe gel-like particles in the transparent solution; with the increase of de-chlorination, the slurry solution became highly viscous. The color quickly changed to orange red, yellowish, and blue with the increase of de-chlorination; finally, solid white particles were visible after an hour with the appearance of the above-mentioned gel-like particles. With the increase of the white solid particles, the slurry solution became less viscous. After a half day of de-chlorination, the current value of the DC power supply dropped to under 0.03 A, which was taken as an indication of ED reaction completion. After the termination of the ED reaction, the hydrous titania precipitates in the main reactor were transferred to a beaker and stirred for several hours. The hydrous titania precipitates were washed in a vacuum filter using DI H 2O and, finally, methyl alcohol solution for the control of particle disintegration. The wet slurries were dried at 70°C overnight, and the crystal phase and microstructure were characterized by XRD and FE-SEM, respectively. During the ED process, the O − ions from H 2O reacted with TiOCl 2 with no further hydrolysis reaction and so the TiO 2 precipitates were able to form. Normally, above 65°C anatase type TiO 2 is formed, but below 65°C rutile TiO 2 is formed. At low temperature, the precipitation reaction is so slow that it forms rutile crystallites, but with an increase of the reaction temperature above 65°C, the precipitation kinetics is rapid enough to form anatase crystallites. 13,14) Through the ED process that is used to remove Cl − ions in aqueous TiOCl 2 solution, sol - gel precipitates were formed and then changed to anatase phase. The experimental details are as follows:
Figure 1(d) shows the Electro-dialysis [ED] process system in which Cl − ions are removed through an anion membrane [AMV]. The sol-gel phase formation of titanium hydroxide in the left chamber is affected by the decrease of [Cl −]. During the ED process, the formation of crystalline and/or non-crystalline phase was influenced by the reaction temperature, which was controlled at < 60°C in order to prevent the degradation of the membrane. The TiOCl 2 solution in the ED reactor showed a color change from red to white with de-chlorination. After ten minutes of ED voltage application, the color changed from orange red to yellow, and then a blue color appeared, as shown in Fig. 1(c). After 30 min, the color changed to white. The phase status changed from sol to gel; then, nano-crystalline particles were confirmed with the removal of Cl −. With the reaction time of dechlorination the quantity of the solid phase increased and the solid paste gradually became crystalline.
2.3. Characterization
XRD [Bruker, M18XCE] was used for the characterization of the dried powders after the termination of the ED process. The microstructure of the sintered body was investigated using FE-SEM [Hitachi, S-4800].
3. Results and Discussion
3.1. Preparation of anatase nanocrystals from TiCl4
In normal NH 4OH precipitation from aqueous solution of TiCl 4, the precipitates show a non-crystalline phase, even after they are heat treated at 100°C for 6 h. 13-15) In order to obtain the crystal phase, the precipitates have to be calcined at above 400°C. If an aqueous solution of TiOCl 2 is stirred at 100°C, anatase crystallites are formed without the formation of the hydrolyzed phase of Ti(OH) 4. Originally, transparent TiCl4 solution is a material that has a large vapor pressure at room temperature and hydrolyzes readily by reacting with water from the air. When a large amount of H2O was added to the transparent TiCl4, a red hydroxide material was formed and then in situ converted into an aqueous TiOCl2 solution of hard yellowish orange color. During the preparation process of the aqueous TiOCl2 solution, there was a dissociation of TiCl4 into the yellow hydroxide and HCl with the addition of a large amount of H2O into TiCl4.
In the ED process, using an anion membrane [AMV, Selemion Asahi Chemical], titania nano-crystallites formed below 60°C. De-chlorination from the hydrolyzed compound of Ti-OH-Cl was done by Diffusion ElectroDialysis [D-ED]. With applied DC [Direct Current] bias, the Cl − ions in the Ti-OH-Cl reactor compartment moved to the surface of the anion membrane, AMV, and diffused to the other side compartment of H 2O, which became an HCl solution due to the increase of [Cl −]. Because of the exothermic reaction between H 2O and Cl − ions, the temperature increased abruptly. A solution temperature above 60°C can deteriorate the carbohydrate polymer membrane [AMV], and so the HCl solution was periodically replaced with pure water in order to cool down the membrane, as shown in Fig. 1(D). If we use a fluorinated ion-exchange membrane such as a Flemion F-8080, [Asahi Glass Japan] or a Nafion membrane [Aldrich], 16,17) the temperature of the ED reaction can be allowed to increase above 60°C. Fluorinated membranes are tougher and more resistant to heat and chemical attack by Cl − ions than the Selemion AMV membrane.
3.2 XRD analysis
Figure 2 shows the XRD patterns of the samples. Anatase phases of nano-crystalline TiO 2 powders were confirmed in the samples, which were prepared by D-ED process of TiO-Cl 2 in aqueous chloride solutions. Small, uniform, and yet size-tunable (5 - 10 nm) anatase titania nano-crystallites were prepared for the samples using a low concentration of TiCl 4 in H 2O (i.e., at molar ratios of TiCl 4/H 2O ≤ 1/15) and a high concentration of TiCl 4 in H 2O (i.e., at molar ratios of TiCl 4/H2O ≤ 1/10) in the temperature range of 40 - 60°C. The formation of anatase phase seems to result from the dechlorination, which is enforced by DC power.
3.3. Microstructure of Anatase powders
Figure 3 show the microstructure of the samples, obtained using FE-SEM, with sample groups of TiO 2-1 and TiO 2-2 for low concentration of TiO 2 and high concentration of TiO 2 in the ED process, respectively. During the ED process, the precipitates for TiO 2-1 were picked up with time from 30 min, 1 h, 2 h, 4 h, 6 h, and 12 h for samples of m001, m002, m003, m004, m005 and m006, respectively. In the TiO 2-2 samples, precipitates were taken out at times of 30 min, 2 h, 6 h, and 12 h for samples of m001, m002, m003, and m004, respectively.
In Fig. 3(A), the nano-crystallite size (3.5 nm) of TiO 2-2 can be seen to be slightly larger than that (3.0 nm) of TiO 2-1 until the de-chlorination reaction has proceeded for two hours. That is, the crystal growth of the nano-crystallites was greatly accelerated by the stronger hydrolysis in the higher concentration sample. 6) It is known that a higher concentration in the solution induces an acceleration of the crystal growth. With the increase of the de-chlorination time from 30 min and 12 h, it can be seen in Fig. 3(b) that the crystal growth of the TiO 2-2 samples has high concentration. The crystalline size of TiO 2-2-m004 is 4.0 nm, as can be seen in Fig. 3(b), which is larger than that (3.5 nm) of TiO 2-2-m001 in Fig. 3(a)
Figure 3(c) shows the crystal growing for TiO 2-1 samples with ED reaction time, and the crystallite sizes are 3.0 nm and 4.0 nm for TiO 2-1-m001 and TiO 2-1-m004, respectively. In Fig. 3(d), for another sample of TiO 2-1, the crystallite sizes are 3.3 nm and 4.3 nm for TiO 2-1-m002 and TiO 2-1-m005, respectively.
Figure 4 shows the crystallite size variation with the ED reaction time for the sample group of low concentration and for the high concentration group, namely groups TiO 2-1 and TiO 2-2, respectively. The variation of the crystal growth rate of the dilute sample, TiO 2-1, is larger than that of the concentrated sample, TiO 2-2. In the D-ED derived crystallites of TiO 2, the formation of the anatase phase seems to be caused by the de-chlorination process. With the appearance of anatase crystallites, the crystal growth is accelerated by the interface reaction between TiO 2 crystal nuclei and the solution ion concentrations, such as the concentrations of [Ti 4+], [Cl −], [O 2−], [H +], and [OH −]. With the decrease of [Cl −], TiO 2 crystal growth is accelerated due to the presence of complex Ti-O-Cl nuclei. This reaction will be enhanced in dilute concentrations of Cl −. In the higher concentration sample of TiO 2-2, the amount of [Cl −] is higher than that in the TiO 2-1 samples, and so this sample shows a low chance of crystal growth. The growth is proportional to the local super-saturation. De-chlorination is enforced by the DC power supply and the ability of the diffusion membrane to perform Cl − ion transfer. In this D-ED process using an AMV membrane, the reaction temperature was controlled so that it stayed below 60°C. The obtained powder slurry was filtered using DI water and ethanol washing. For the dry sample powders, the presence of anatase crystallite was confirmed by XRD analysis, as can be seen in Fig. 2.
3.4. Discussion
Figure 4 shows the typical crystal growth of TiO 2 with the removal time of Cl − ions during the de-chlorination of precipitates in the D-ED reactor after solution precipitation. The crystal growth of TiO 2 in the Ti-OH-Cl solution increased with the de-chlorination of the slurry solution. Of course, there can be several other factors involved such as the Cl − ion detachment rate from the Ti-OH-Cl complex compound, the ion transfer rate in H 2O, and the Cl − ion diffusion rate through the AMV membrane. Details on the relative influence of each factor 18-23) will be determined and provided in another report. In the normal hydrolysis of TiCl 4, precipitates of the Ti-OH-Cl compound are formed. In this D-ED process to remove Cl − ions in the aqueous solution of the Ti-OH-Cl compound, the elimination of Cl − ions in the aqueous solution is quickly accomplished. In the next step, the Cl − ions in the Ti-OH-Cl compound are gradually detached and transferred to the anion reactor through the anion membrane, AMV, by the application of DC current. The existence is confirmed of octahedral hydroxochloro complexes of the type [Ti(OH) aCl b(OH 2) c] (4−a−b)+, where a + b + c = 6, and a and b depend on the acidity and the concentration of Cl − in the solution. 12) In this D-ED process, the DC power enforces the detachment of Cl − ions in the Ti-OH-Cl complexes; this may be the rate limiting step in the transfer of Cl − ions to the anion compartment through the AMV membrane. With the removal of Cl − ions, the Cl − ion concentration decreases and the reaction requires higher enforcing power for Cl − ion detachment in the complex. In our experimental design, we decided that the removal of Cl − ions had finished when the current value was under 0.03 A in the DC power supply.
4. Conclusions
Using the D-ED process, mono-dispersed ultrafine TiO2 particles with diameters of 3 - 4.5 nm were obtained through de-chlorination from aqueous TiOCl2 solution with a 0.5M Ti4+ concentration prepared by diluting TiCl4 in a homogeneous spontaneous precipitation process. The dechlorinated precipitates were dried at 60°C; through analysis using XRD and FE-SEM, the phase was found to be anatase nano-crystallites. With increased de-chlorination time, the crystal growth rate of TiO2 in the lower concentration sample of Ti4+ ions was found to be larger than that of the higher concentration sample of Ti4+ ions.
Acknowledgments
This research was supported by the general research support program of the National Research Foundation (NRF), funded by the Korean Government (2013R1A1A2A10058275).
Fig. 1
(a) Experimental design of hydrolysis process for TiCl4 solution. The inert atmosphere was controlled through a glove box and a Schlenk line. The main round flask was in an ice bucket. (b) The aqueous solution of TiCl4 was hydrolyzed with DI H2O in the flask, showing a red-yellowish color. (c) Reactor for ED process. The electrode is a Pt-coated Ti alloy. Through the AMV membrane, Cl− ions are transferred to the right side compartment by DC power. (d) Total experimental design for the D-ED process. 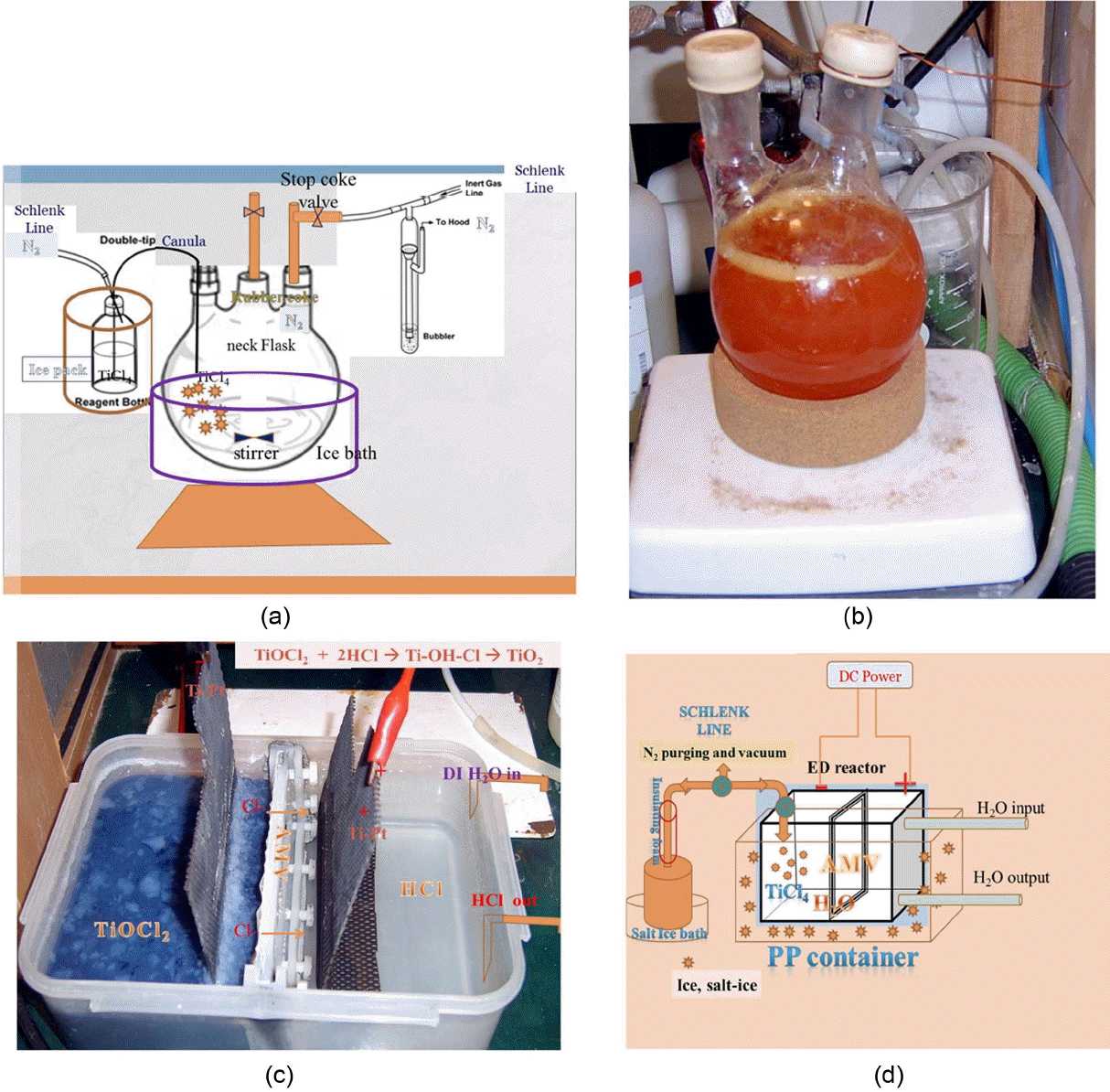
Fig. 2
XRD analysis for TiO2-1 and TiO2-2 samples for the higher concentration and the dilute concentration, respectively. 
Fig. 3
Photographs of the samples of high concentration, TiO2-1, and low concentration, TiO2-2. (a) Crystal growth in TiO2-1 with ED times of m001 and m005, and crystal growth in TiO2-2 with ED times of m001 and m003. (b) Crystal growth in TiO2-2 with ED times of m001, m002, m003, and m004. (c) Crystal growth in TiO2-1 with ED times of m001, m003, m004, and m006. (d) Crystal growth in TiO2-1 with ED times of m002, m003, m005, and m006. 
Fig. 4
Crystal growth with time during ED process. 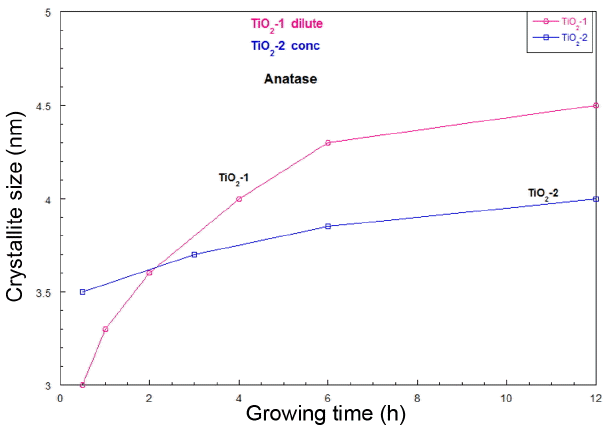
REFERENCES
1. AL. Linsebigler, GQ. Lu, and JT. Yates, “Photocatalysis on TiO2 Surfaces: Principles, Mechanisms, and Selected Results,” Chem Rev, 95 [3] 735-58 (1995).  2. B. O’Regan, and M. Gratzel, “A Low-Cost, High-Efficiency Solar Cell Based on Dye-Sensitized Colloidal TiO2 Films,” Nature, 353 737-40 (1991).  3. LD. Birkefeld, AM. Azad, and SA. Akbar, “Carbon Monoxide and Hydrogen Detection by Anatase Modification of Titanium Dioxide,” J Am Ceram Soc, 75 [11] 2964-68 (1992).  4. H. Wang, Y. Wu, and BQ. Xu, “Preparation and Characterization of Nanosized Anatase TiO2 Cuboids for Photocatalysis,” Appl Catal, B, 59 [3] 139-46 (2005).  5. SD. Park, YH. Cho, WW. Kim, and S-J. Kim, “Understanding of Homogeneous Spontaneous Precipitation for Monodispersed TiO2 Ultrafine Powders with Rutile Phase around Room Temperature,” J Solid State Chem, 146 [1] 230-38 (1999).  6. L. Vechot, JEH. Buston, J. Kay, GA. Round, S. Masharani, GA. Tickle, and R. Rowlands, “Experimental Study of the Liquid Phase Hydrolysis Reaction in Titanium Tetrachloride,” Hazards XXII, Symposium Series, 156 238-45 (2011).
7. MC. Chang, “Yttrium-Stabilized Zirconia Particles Prepared Using Electro-dialysis of (Zr,Y)OCl2 Aqueous Solution,” J Korean Ceram Soc, 51 [5] 466-71 (2014).  8. MC. Chang, and R. DeLong, “Calcium Phosphate Formation in Gelatin Matrix Using Free Ion Precursors of Ca2+ and Phosphate Ions,” Dent Mater, 25 [2] 261-68 (2009).  9. Y. Chen, A. Lin, and F. Gan, “Preparation of Nano-TiO2 from TiCl4 by Dialysis Hydrolysis,” Powder Technol, 167 [3] 109-16 (2006).  10. SJ. Kim, SD. Park, YH. Jeong, and S. Park, “Homogenous Precipitation of TiO2 Ultrafine Powders from Aqueous TiOCl2 Solution,” J Am Ceram Soc, 82 [4] 927-32 (1999).  11. SJ. Kim, CH. Jung, SD. Park, SC. Kwon, and S. Park, “Preparation of Crystalline TiO2 Ultrafine Powders from Aqueous TiCl4 Solution by Precipitation Process,” J Korean Ceram Soc, 35 [4] 325-32 (1998).
12. AD. Paola, G. Cufalo, M. Addamo, M. Bellardita, R. Campostrini, M. Ischia, R. Ceccato, and L. Palmisano, “Photocatalytic Activity of Nanocrystalline TiO2 (Brookite, Rutile and Brookite-based) Powders Prepared by Thermohydrolysis of TiCl4 in Aqueous Chloride Solutions,” Colloids Surf, A, 317 [1-3] 366-76 (2008).  13. Q-H. Zhang, L. Gao, and J-K. Guo, “Preparation and Chracterization of Nanosized TiO2 Powders from Powders from Aqueous TiCl4 Solution,” Nanostruct Mater, 11 [8] 1293-300 (1999).  14. JH. Lee, and YS. Yang, “Effect of HCl Concentration and Reaction Time on the Change in the Crystalline State of TiO2 Prepared from Aqueous TiCl4 Solution by Precipitation,” J Eur Ceram Soc, 25 3573-78 (2005).  15. T-H. Wang, AM. Navarrete-Lopez, S. Li, DA. Dixon, and JL. Gole, “Hydrolysis of TiCl4: Initial Steps in the Production of TiO2,” J Phys Chem A, 114 [28] 7561-70 (2010).  16. J. Wang, C. Xu, M. Taya, and Y. Kuga, “A Flemion-Based Actuator with Ionic Liquid as Solvent,” Smart Mater Struct, 16 [2] S214-19 (2007).  17. K-L. Huang, TM. Holsen, and JR. Selman, “Anion Partitioning in and Diffusion through a Nafion Membrane,” Ind Eng Chem Res, 42 [9] 3620-25 (2003).  18. W. Wang, and WR. Hu, “Concentration Distribution in Solution Crystal Growth: Effect of Moving Interface Conditions,” J Cryst Growth, 203 [1-2] 227-33 (1999).  19. P. Pong Chang, and MD. Donohue, “A Kinetic Approach to Crystallization from Ionic Solution, I. Crystal Growth,” J Colloid Interface Sci, 122 [1] 230-50 (1988).  20. P. Pong Chang, MD. Donohue, and JL. Katz, “Kinetic Approach to Crystallization from Ionic Solution II. Cystal Nucleation,” J Colloid Interface Sci, 122 [1] 251-65 (1988).  21. P. Pong Chang, and MD. Donohue, “The Effect of Complex Ions on Crystal Nucleation and Growth,” J Colloid Interface Sci, 126 [2] 579-91 (1988).  22. O. Sohnel, “The Mechanism of Crystallization; A Revision of Cencepts. Comment,” Mater Res Bull, 9 [4] 489-94 (1974).  23. SPF. Humphreys-Owen, “Crystal Growth from Solution,” Proc R Soc London, Ser A, 197 [1049] 218-37 (1949). 
|
|















