1. Introduction
Calcium carbonate (CaCO3) is an abundant mineral in nature; making up about 5% of the Earth’s crust in the form of limestone.1) It is an important building material in living organisms (e.g., bones, teeth, and shells); moreover, it can be found in several industrial applications. Calcium carbonate is used as a coating pigment in paper, rubber, and adhesives; as filler in light weight plastics and concrete; for environmental pollution control and remediation in flue gas and water treatment; in fertilizers and animal feed as calcium supply; and in glass, ceramics, cosmetics, and hygienic products.2)
In the past decades, calcium carbonate (CaCO3) has attracted much attention due to its abundance in nature, and as an attractive model mineral for laboratory studies.3-5) It is well known that there are three anhydrous crystalline phases of CaCO3: calcite, aragonite, and vaterite. Calcite is thermodynamically the most stable, aragonite is metastable, and vaterite is the least stable and easily transforms into one of the other two phases.6) In practice, the stability of these polymorphic species depends mainly on temperature. 7-8) However, the previous experiments were performed mainly at ambient temperatures.
The deposition of solid particles from the liquid phase through a precipitation reaction is a simple and reliable method for obtaining nanostructures with good control of the particle characteristics. Several studies have been published regarding the synthesis of metal oxides, hydrous oxides, and hydroxide particles by precipitation from aqueous solutions.9-10) In this study, the newly developed liquid-liquid simultaneous injection method usually provides a mixture of polymorphs because of the three polymorphs (calcite, aragonite and vaterite). In order to induce the crystallization of aragonite (the high pressure polymorph) selectively, the precipitation conditions, such as temperature, pH, feeding order, stirring, and reaction time must be carefully controlled.
In the present work, crystallization of CaCO3 at different temperatures, pH, and different reaction times was investigated. Nano whisker-type aragonite of high aspect ratio was produced under various conditions. This study provides new understanding of the crystallization of CaCO3 in complex systems.
2. Experimental Procedure
The starting materials used in this study were CaCl2 and Na2CO3 (95 and 99%, Junsei Company, Japan).
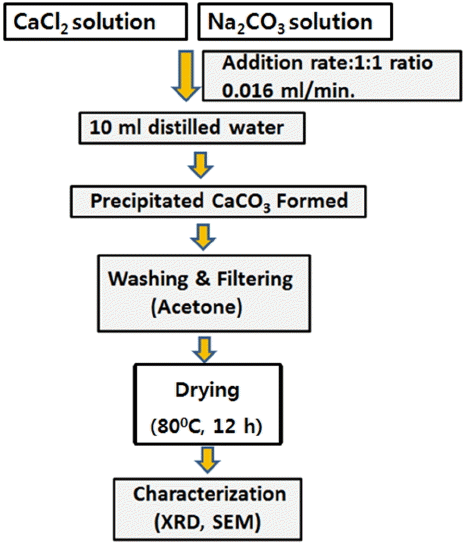
Nano aragonite whiskers was synthesized by simultaneous injection of 0.1M solutions of CaCl2 (1.5 mL) and Na2− CO3 (1.5 mL) into a glass reactor containing 10 mL of double-distilled water at a rate of 0.016 mL/min and reaction temperature of 25 - 50°C. In the experimental process a magnetic stirrer was used to agitate the reaction medium in a glass reactor. The primary reactant solutions CaCl2 and Na2CO3 were simultaneously injected into the reactor. The precipitate was aged for 0.5 - 1.5 h, then filtered and dried at 80°C for 12 h, as shown in Fig. 1. The resulting product was nano aragonite whiskers. The general mechanism involved in the Na2CO3 and CaCl2 reactions can be expressed as follows:
Reaction Mechanism:
Supersaturation (SI) of the solution with respect to calcium carbonate,
where (Ca2+) and (CO32−) are the activities of calcium and carbonate ions in the solution, respectively, and Ksp is the thermodynamic solubility of the aragonite product.
During the solution process experiments, metastable crystalline forms of CaCO3 such as vaterite were not identified in the X-ray diffraction spectra.
3. Results and Discussion
The initial concentration of calcium ions plays an important role in the synthesis of nano aragonite whiskers by the simultaneous injection method, at different temperatures.
3.1. Effects of temperature
Temperature is a key factor in the formation of aragonite whiskers. It is important to point out that a small difference in reaction temperature can result in dramatic changes in the crystal phase while other parameters are kept constant. A hydrothermal hot processing technique was developed to solidify the aragonite form of calcium carbonate; therefore, its application as solid material was increased.11) Temperature and aging time affects the formation of polymorphs. Some researchers reported the addition of calcium nitrate and sodium carbonate without controlling the pH, calcium carbonate was formed by indirect precipitation. The composition and high fraction of aragonite was dependent on the temperature > 50°C, with digestion time of 0.1 h.12)
Many researchers have investigated the effects of temperature on the formation of aragonite whiskers/needles.13- 24) Different types of aragonite crystals were synthesized by combining aqueous solutions of Na2CO3 and CaCl2 under ambient reaction conditions. A plausible mechanism was proposed for the formation of aragonite based on the phase transformation behavior of calcium carbonate during synthesis. This preparation technique involved simultaneous injection of aqueous solutions of Na2CO3 and CaCl2 at different temperatures without any additives. This led to the formation of aragonite with different shapes. From these results, it was seen that three reaction parameters showed significant effect on the morphology of particles in the aragonite phase namely (i) the temperature of the reaction, (ii) the reaction time, and (iii) the effect of pH. These aragonite crystals were found to be stable in solid states for a long time.25)
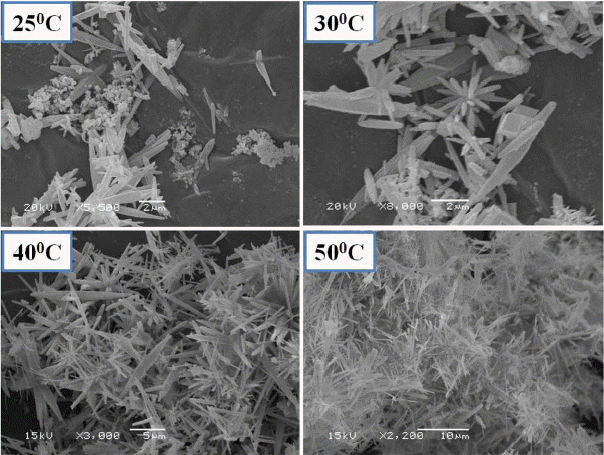
The present work revealed that nano aragonite whiskers were synthesized by combining Na2CO3 and CaCl2 solutions injected (1:1 ratio) into distilled water (liquid-liquid reaction) at different temperatures (25, 30, 40 and 50°C). That pure uniform nano aragonite needles (100-200 nm) were synthesized with high aspect ratio (30) at 50°C, is clearly observed from the XRD analysis (Fig. 2) and scanning electron microscope images. Fig. 3 shows the morphology of nano whisker aragonite. By decreasing the temperature to 40, 30, and 25°C, a mixture of aragonite and calcite phases were obtained. Based on the experimental results, the reaction temperature for each of the CaCO3 polymorphs should play a key role in determining which of the polymorphs of CaCO3 is formed.
3.2. Effect of reaction time
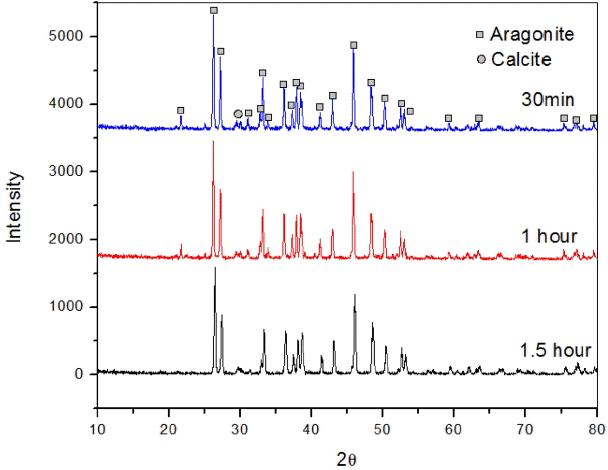
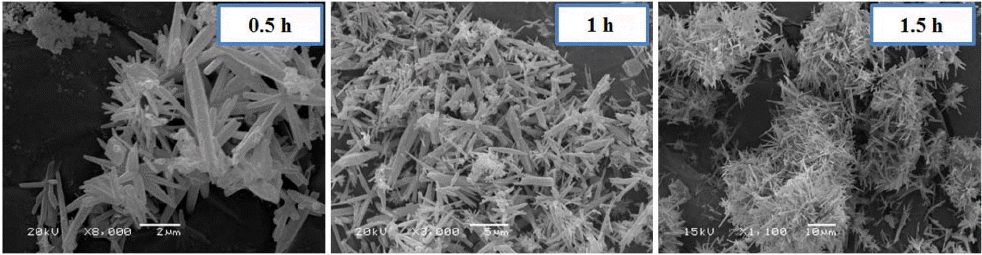
Time also plays a key role in the formation of aragonite whiskers. In the present study, to analyze the different effects of time on the simultaneous injection process, we observed the synthesis of whisker-type nano aragonite while injecting Na2CO3 and CaCl2 into aqueous solution at 50°C, with different duration of reaction (0.5, 1.0, or 1.5 h). We obtained pure nano aragonite whiskers with 1.5 h reaction time by the solution process, which was confirmed by the XRD results (Fig. 4) and scanning electron microscopy (SEM) images. Fig. 5 shows the morphology of the nano aragonite whiskers.
The reaction times of 0.5 and 1.0 h after simultaneous injection produced a mixture of aragonite and calcite phases. Based on the experimental results, the calcite phase transformed into aragonite when the reaction time was extended to 1.5 h, the reaction time of CaCO3 polymorphs should play a key role in the simultaneous injection process.
3.3. Effect of pH
The pH of the reaction solution is an important factor in the formation of the desired phase of aragonite. Pure aragonite was obtained at pH 10; when the pH was greater than 12, calcite was obtained.26) From the parametric variation study on the synthesis of aragonite, it was concluded that pH < 11 at ~ 7°C, supported the formation of calcite and aragonite, but that nearly pure aragonite was obtained at 58°C with pH < 10.27) Highly oriented aragonite particles were found in the nacre layers of mollusk shell, and recently, aragonite rods were also obtained at pH ranging from 1.5 to 6.9, without bio or organic macromolecules.28)
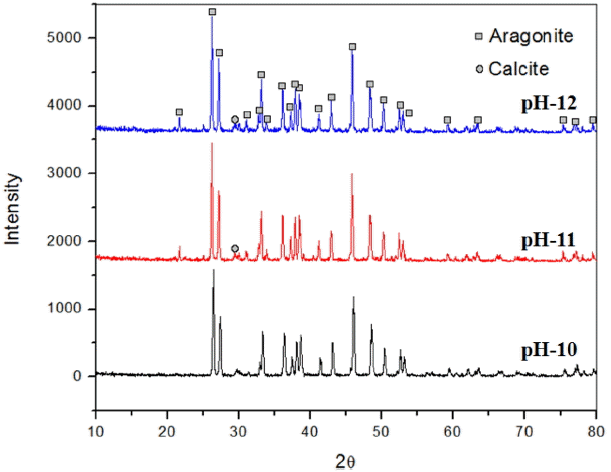
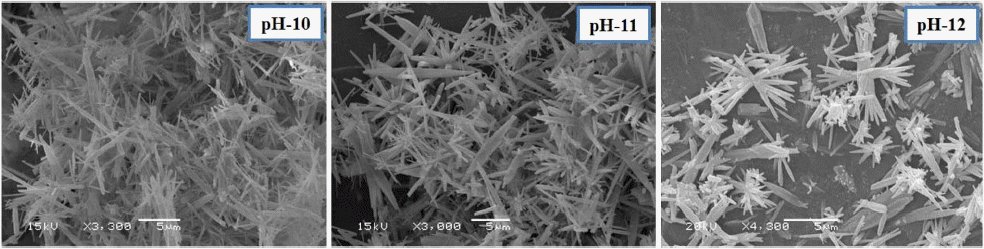
The results from the present study revealed that nano aragonite whiskers were synthesized by combining of Na2− CO3 and CaCl2 solutions injected simultaneously (1:1 ratio) into distilled water (liquid-liquid reaction) at different pH (10, 11, or 12). Pure uniform nano aragonite needles (100 - 200 nm) were synthesized with high aspect ratio (30) at pH 10, which is clearly observable from XRD data shown in Fig. 6, and Fig. 7 shows the morphology of nano whisker aragonite in scanning electron microscopy images. By increasing the pH from 11 to 12, a mixture of aragonite and calcite phases was obtained. Based on the experimental results, pH 10 is most suitable for synthesis of pure aragonite whiskers.
3.4. Applications
Calcium carbonate (CaCO3) is one of the most popular mineral fillers used in the plastics industry. It is widely available around the world, easy to grind or reduce to a specific particle size, compatible with a wide range of polymer resins, and economical. As an additive in plastic compounds, CaCO3 helps decrease surface energy and provides opacity and surface gloss, which improves the surface finish. In addition, when the particle size is carefully controlled, CaCO3 helps increase both impact strength and flexural modulus (stiffness).29)
4. Conclusions
The production of nano particulate, precipitated calcium carbonate (PCC), by simultaneous injection was performed in a glass reactor chamber. The simultaneous injection method was demonstrated with a chosen range of process parameters (temperature, reaction time, and pH). From these, aragonite particles/crystals with different characteristic morphologies were produced. Because of the large number of physico-chemical processes, taking place during the CaCl2 and Na2CO3 reactions (dissolution and homogeneous nucleation of CaCO3), it is difficult to precisely predict the PCC properties. A large number of experiments are needed in order to obtain a product with exactly the desired properties. Therefore, an empirical approach based on experimental design techniques was employed to identify the effects of the chosen process parameters on the PCC morphology (specific shape of calcium carbonate).
A multiple experimental analysis of the obtained data suggests that temperature, reaction time, and pH play key roles in determining the nano PCC morphology. At lower temperatures, calcite can be produced, whereas higher temperature is suitable for obtaining nano aragonite PCC. These results emphasize the role of the liquid-liquid phase interface on the physical chemical properties of nano precipitated calcium carbonate.
Finally, this study demonstrated that the temperature, reaction time, and pH have significant effects on the average particle size, on precipitation, and on the morphology of the calcium carbonate crystals produced. Facile synthesis of nano aragonite whiskers was possible by liquid-liquid reaction using simultaneous injection. This process was used to control the experimental parameters to produce particles of average size from 100 - 200 nm. The use of the aragonite form of precipitated calcium carbonate as a functional mineral filler for polypropylene is now growing. This is mainly due to its whisker/needle like morphology (similar to the shape of wollastonite needles). The results presented here demonstrate that nano aragonite crystals with strong potential for industrial applications, including as filler for light weight plastics in motor industries and paper industries, could be synthesized by simultaneous injection solution process under optimized conditions. The major advantage of this proposed method for obtaining nano aragonite needles is that it consumes less energy and offers cost benefits, being free of additives and not requiring template systems.