1. Introduction
Activated carbon is one of the most important industrial carbon materials. The use of activated carbon for adsorption treatment was first recorded more than two hundred years ago. Activated carbon adsorbers are now widely employed for product purification (such as sugar refining, food processing, and the production of water) and pollution control (such as water and wastewater treatment and the removal of volatile organics from industrial process gases). Activated porous carbons have been widely used in adsorbent or gas storage,1,2) treatment of pollution in water 3) and as catalyst support;4) they have also been used in electronic devices such as electrodes of electrical double layer capacitors.5,6)
To the best of our knowledge, the adsorption capacity of AC is mainly determined by the specific surface area, pore size distribution, and surface functional groups.7,8) However, for other applications, the ash content and the high temperature resistance remain amongst the most important properties of the adsorbent used. High temperature stability is particularly important during reactivation and in gas-phase adsorption applications,9,10) such as stack gas scrubbing and hydrogen purification, amongst others, due to the high temperature of the process gases. Therefore, it is necessary to fabricate a novel active carbon based material with high adsorption ability and high ignition temperature because among the processes of transport and storage the use of bare AC can easily pose a spontaneous combustion hazard due to its low self-ignition temperature.11)
Recently, siliceous zeolite has been reported by Niu et al. to exhibit excellent thermal and hydrothermal stability, which allows it to maintain its structure perfectly even in 100% steam at 1000°C.12) Meanwhile, zeolites are an interesting kind of crystalline aluminosilicate mineral with high surface area and high adsorption ability; as such they are commonly used as adsorbents.13) Yoneyama et al.14,15) studied the role of porous solids with different adsorption capacities; studied materials in include zeolites, activated carbon, and silica, and alumina for the photocatalytic degradation of propionaldehyde. They concluded that zeolite structures exhibit important physicochemical properties compared to those of other adsorbents such as activated carbon. Zeolites Y and ZSM-5 are some of the most commonly ordered structures used in photocatalytic studies, including studies on the removal of nitrogen oxides,16,17) the photoinactivation of pathogenic microorganisms,18) CO2 photoreduction,19) or the elimination of toluene and benzene.20)
Activated carbon fibers (ACFs) and granular activated carbons (GACs) are two of the most widely used activated carbon materials. However, spherical activated carbons (SACs) have received considerable recent attention for their various potential advantages over ACFs and GAC; these advantages include extremely low resistance to liquid diffusion, higher adsorption efficiency, better mechanical properties, and more resistance to abrasion.21)
Hence, considering the advantage of activated carbon and zeolite, we have here adopted a simple method for the synthesis of zeolite combined activated carbon spherical samples. The textural properties of zeolite-SACs were characterized by BET, XRD, SEM, EDX, and pressure; benzene and iodine adsorption tests, bactericidal effect test, and ignition temperature test were also performed.
2. Experimental Procedure
2.1. Materials
The main material was coal based activated carbon with a size of 100 ~ 400 mesh; charcoal was provided by Neven Co., Ltd. (Korea). ZSM-5 zeolites with main content of SiO2 and Al2O3 were purchased from Paik Kwang Chemical Co., Ltd. (Korea). Phenolic resin (PR) as the main bonding agent was purchased from Kangnam Chemical Co., Ltd. (Korea). Dark brown sugar and potato starch as auxiliary binder were purchased from CJ Beksul and SeungJin Food. Co., Ltd. (Korea). Alcohol (95%), which was used as a dispersing agent, was purchased from Samchun Pure Chemical Co., Ltd. (Korea).
2.2. Preparations of zeolite combined activated carbon spheres
In order to form the zeolite-SACs, a shaped mould, shaker sieve, and homemade shaker were used. First, two kinds of binder material, dark brown sugar, potato starch, and a calculated amount of PR were dissolved in a small amount of water and an appropriate amount of alcohol, respectively. After adequately mixing these materials with active carbon, the mixture was then put into shaped moulds to form agglomerates. To make spheres of these agglomerates, a shaker and a sieve with size of 30 ~ 50 mesh were used with a shaking rate of 50 round/min at room temperature. After shaking for 30 min, the agglomerates formed spheres; they were then dried in the activated carbon powder at 300°C and heat treated at 600°C for 2 h. Then, the spherical agglomerates were heated with CO2 at a flow rate of 150 mL/min at about 800°C for 2 h with a heating rate of 10°C/min to form activated zeolite-SACs. The preparation conditions and the sample codes are shown in Table 1.
2.3. Characterization
The composite zeolite-SACs were characterized by scanning electron microscopy (SEM) using a JSM-5200 JOEL equipped with an X-ray detector for energy dispersive X-ray analysis (EDX). XRD patterns were obtained at room temperature with a Shimata XD-D1 device (Japan) using CuKα radiation. The BET surface area of the SACs was measured using a Quantachrome surface area analyzer (Monosorb, USA).
2.4. Pressure drop measurement
Pressure drop of the zeolite-SACs (AZP4,6,8) sample was measured using a circulating pipe with an inside diameter of 27 mm and a length of 12 m. The samples in the tank were introduced into a pipe by a pump (2NE-20A) with the capacity of 3.5 m3/h. The pressure drop was taken out by changing the velocity of the range of 0.25-1.7 m/sec in the pipe. The Hazen-Williams equation for calculating the pressure drop due to frication for a given pipe diameter and flow rate is as follows:22)
where
2.5. Benzene and Iodine removal determination
Liquid phase adsorption of benzene: batch experiments were performed to study the adsorption capability of the zeolite-SACs (AZP4,6,8) produced for benzene in solution. 2 g of each zeolite-SAC (AZP4,6,8) sample were weighed into different 500 ml conical flasks. 20 ml of benzene was measured from the original mixture and poured into each 50 ml conical flask and agitated at a stirring speed of 200 rpm to ensure intimate contact of the adsorbent and the solute in the solution. Each conical flask was observed for 20 mins and filtered with filter paper. A portion of the filtrate from each flask was poured into a cuvette and the concentration of benzene in the solution was measured using a spectrophotometer. Using the sodium thiosulfate volumetric method, the amount of iodine was determined based on ASTM D 4607-8617. The zeolite-SACs (AZP4,6,8) were passed through a 325 mesh screen. Standard iodine solution was added over the zeolite-SACs (AZP4,6,8) (0.5 g) and, after an equilibration time of 30 s, the residual iodine concentration was determined by titration with standard sodium thiosulfate with starch as an indicator. The amount of iodine was defined as the quantity of iodine adsorbed (in mg/g of carbon) at a given residual iodine concentration.
2.6. Ignition temperature detection
The ignition temperature values of the AZP6 series spherical samples with different amounts of PR (10 ~ 35%) and fixed amounts of zeolite (6%) were detected using a 1000°C infrared thermometer (TM-969, Taiwan). The AZP6 series spherical samples (AZP6-10, AZP6-20, AZP6-30, AZP6-35,) were exposed to air and heated in a calcination furnace with a heating rate of 10°C/min, while the set highest temperature was 700°C. The ignition temperature was recorded when these samples started burning with a slight bit of flame.
2.7. Antibacterial effects
For the detection of antibacterial effects, the AZP6 series spherical samples were treated with different weight amounts of Ag+ (6, 8, 10%) and a fixed amount of zeolite (6%); the treated samples were used as a bactericide and were denoted as Ag6, Ag8, and Ag10. Employing the halo test proposed by the Berman method,23) the bactericidal activities against B. Subtilis, S. Epidermis, E. Coli, and S. Aureus were examined in a cultivated culture medium. For quantitative analysis of the bactericidal effects, the flask shaking method was employed.24) In our previous studies, 25,26) the bactericidal activity levels of Ag6, Ag8, and Ag10 materials against B. Subtilis, S. Epidermis, E. Coli, and S. Aureus were investigated in detail using the flask shaking method. The bactericidal activity against the strain used herein was examined in a cultivated culture medium. For the test, 300 mL of prepared Trypticase Soy Broth (TSB badge, ca. 120°C, 15 min) was first sterilized. Then, each badge strain was cultivated for 24 h under conditions of constant humidity, at a temperature of 37°C. After culturing, a phosphate buffer solution was added to the cultured solution. The strain in the solution was then counted again. The Ag6, Ag8, and Ag10 samples were then dispersed into the counted strain solution, both with and without sunlight. After dispersion and irradiation, the number of bacteria was counted as a function of time. The process was carried out again after 120 min under constant humidity and temperature.
3. Results and Discussion
3.1. Structure analysis and morphology
Phase identification was carried out by XRD. The solid lines in Fig. 1 show the XRD patterns of the prepared spherical samples: (a) AZP4, (b) AZP6, and (c) AZP8. Specifically, the zeolite skeleton is basically retained, showing main characteristic peaks at 22.5-25°, 26.5°, 27.5°, 31.5°, 35.5°, 43°, and 45°, which are in good agreement with the orthorhombic phase of ZSM-5 zeolite;27,28) the typical characteristic peaks of SiO2 and Al2O3 appear at 22.5° and 32-45°, respectively.29) The intensity of the peak for SiO2 and Al2O3 in sample AZP6 exhibits a value higher than that of AZP4 and AZP8; it can be speculated that the amount of zeolite used in AZP6 is relatively appropriate. According to previous studies, the appropriate amount of zeolite used in composite materials will lead to excellent gas removal activity.16,17)
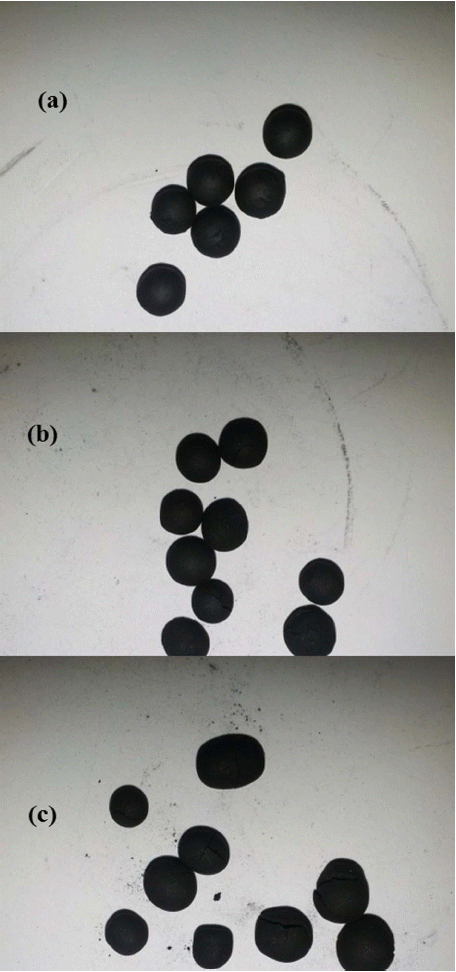
Figure 2 provides photographs of the prepared spherical samples: (a) AZP4, (b) AZP6, and (c) AZP8. From Fig. 2, we can see that after heat treatment at 600°C for 2 h, a crack is formed by the change of the amount of zeolite in the zeolite-SACs. The crack is increased by the increase of the amount of zeolite. This crack cannot be observed in AZP4, which had the lowest amount of zeolite, while sample AZP4 had a few cracks after carbonization and activation at high temperature. The cracks of AZP8, which had the greatest amount of zeolite, were very well formed due to the weak binding force between the binder material and zeolite, AC because the phenolic rosin is brittle and not resistant to high temperature; brittleness often results in wear loss and fade below the temperature of 350°C.30) In order to detect the relationship between the amounts of zeolite and AC, morphology was analysis by SEM followed.
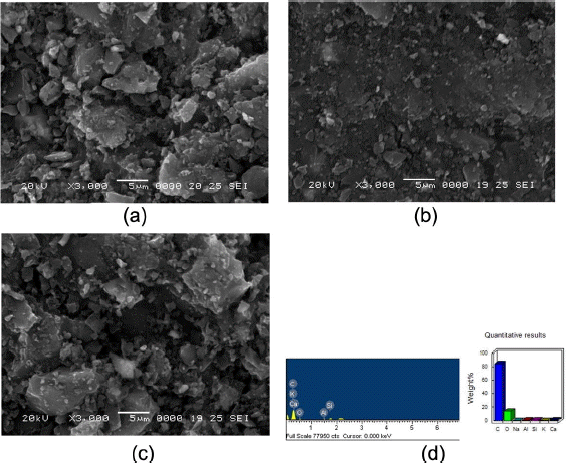
The SEM images of the zeolite-SACs (AZP4, AZP6, AZP8) are provided in Fig. 3. From the SEM image in Fig. 3(a), it can be seen that the zeolite particles were deposited on the surface of the activated carbon with a little aggregation. This indicates that a small amount of zeolite and a relatively larger amount of AC powder cannot homogeneously mix with the binder materials, though the photograph of AZP4 shows that this sample has the best mixing. Because most of the AC powder was agglomerated, it formed a lot of bulk areas inside of the sphere sample. With increasing amounts of zeolite and decreasing amounts of AC, the morphology of the prepared spherical samples became better, as shown in Fig. 3(b). This can be taken to indicate the excellent adsorption property of zeolite and AC, both of which promote adsorption binder materials in the mixing process and thus lead to a better morphology.7,14) However, when the amount of zeolite was increased to 8% in AZP8, a result can be observed that is similar to that for sample AZP4: more and larger bulks have been generated, as is shown in Fig. 3(c). As a result, the specific surface areas of AZP4, AZP6, and AZP8 were found to be 1194 m2/g, 1011 m2/g, and 982 m2/g; these decreased results may presumably have been generated by the increased amount of zeolite and not by the homogeneous mixing station.
EDX analysis was carried out to probe the composition and element weight percent of sample AZP6, with results shown in Fig. 3(d). The spectra showed the main elements to be Ka, Ca, O, Al, and Si, with a strong C peak. O, Al, and Si were confirmed as the main componential elements from SiO2 and Al2O3 in zeolite. These results indicate that after carbonization and activation at high temperature, the composite of zeolite-SACs was not destroyed due to the excellent thermal and hydrothermal stability of zeolite.12)
3.2. Pressure drop of zeolite-SACs
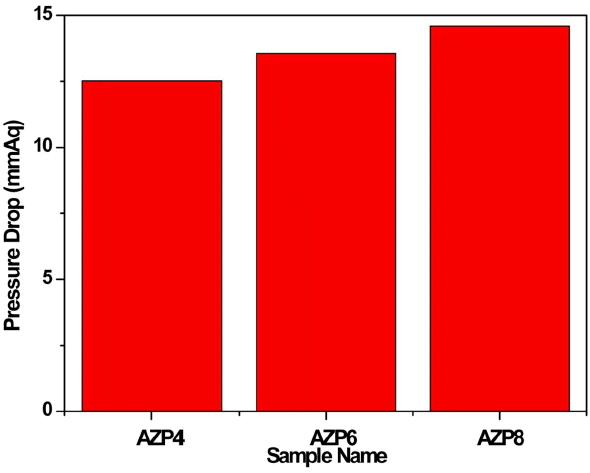
Fig. 4 shows the pressure drop of the zeolite-SACs (AZP4, AZP6, AZP8) after activation. We can clearly see that the pressure drop of the zeolite-SACs decreased with increasing of the amount of zeolite from 4% to 8%. This can indicate that the formation of a homologous composite is an important factor for the pressure drop. As can be seen in Fig. 2, AZP4 has the best formation of spherical shapes, and can be considered to have the lowest pressure drop, which is in good agreement with the results shown in Fig. 4.
3.3. Benzene and iodine removal capability of zeolite-SACs
The results for the benzene removal effect using zeolite-SACs are shown in Fig. 5(a). Almost all of the samples exhibited significant benzene removal efficiency, with values ranging from about 23 to 24.9%. The results obtained for sample AZP6, especially, show that the high removal efficiency contributed to the removal of liquid phase benzene. Fig. 5(b) shows changes of the iodine adsorption capability of the zeolite-SACs before and after activation. Compared with the iodine adsorption capability of the zeolite-SACs before activation, the iodine adsorption capability decreased after activation, from 980 to 920 and 888 mg/g, while the iodine adsorption capability of the zeolite-SACs before activation increased from 412 to 423 and 429 mg/g. As described above, the spherical agglomerates were heated with CO2 at a flow rate of 150 mL/min at about 800°C for 2 h with a heating rate of 10 °C/min to form activated zeolite-SACs. Opposite results may have be caused by the higher heat-treatment temperature of about 800°C; some amount of resin will be thermal decomposed and thus exhibit a drop of its viscous force.31) According to previous studies, for pure activated carbon, adsorption in the liquid phase is a more complicated process than gas or vapor-phase adsorption.32) The obviously different removal capabilities of AZP6 for benzene and iodine adsorption, observed in Figs. 5(a) and (b), can be attributed to the better volatility of benzene than that of iodine.
3.4. Ignition temperature of AZP6 series spherical samples
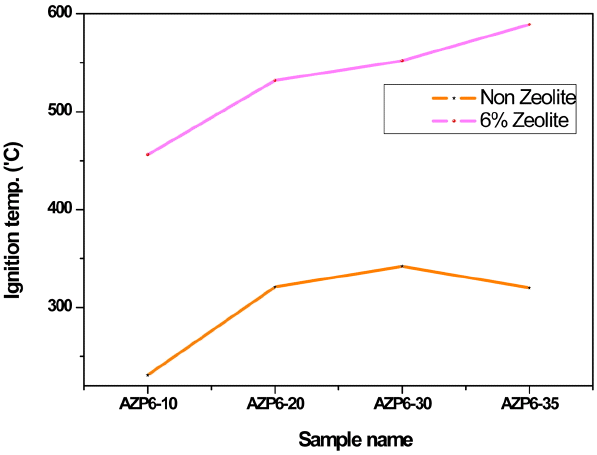
Figure 6 shows the ignition temperatures of the AZP6 series spherical samples with different amounts of PR (10 ~ 35%) and fixed amounts of zeolite (6%). Differences of ignition temperature for the AZP6 series spherical samples with and without zeolite modification can be easily observed in Fig. 6. Compared with the samples prepared using the least amount of 10% PR with and without zeolite modification, the ignition temperature of AZP6-10 was found to increase remarkably from 231 to 456°C due to better thermal and hydrothermal stability of zeolite.12) On the other hand, with an increase of the amount of PR from 10 to 35%, the AZP6 series spherical samples with zeolite modification were found to exhibit continuous enhanced ignition temperature from 456 to 589°C. However, without zeolite modification and when the amount of PR was increased from 30 to 35%, the ignition temperature of the AZP6 series spherical samples was found to decrease from 342 to 320°C.
3.5. Antibacterial activity
The antibacterial activity of the Ag6, Ag8, and Ag10 series spherical samples against B. Subtilis, S. Epidermis, E. Coli, and S. Aureus was investigated due to its easy availability and for its ubiquitous presence. For the purpose of comparison, we used antibiotic Kanamycin as a standard, given in the culture media of the bacteria. Fig. 7(a), (b) shows the excellent bactericidal effects of B. Subtilis and S. Epidermis. In Fig. 7(a), the Ag10 sample can be seen to restrict the growth of bacterial colonies and form a clear zone with an approximate diameter of 1.1 cm, while the approximate diameters for the Ag6 and Ag8 samples was in the range of 0.75 - 0.8 cm. With increasing of the amount of Ag+ from 6% to 10%, the approximate diameter of the clear zone increased from 1.1 to 1.35 cm, as shown in Fig. 7(b), while the approximate diameter of the clear zone created by Ag8 decreased to 1 cm. From the obtained results, it can be seen that the use of a proper amount of Ag+ in the composites can promote a better bactericidal effect.33) According to the results shown in Figs. 7(c) and d, a clear but smaller inhibition zone around 0.9 cm and 0.55 cm for Ag10 was observed, indicating the existence of the slight antibacterial effect of E. Coli and S. Aureus of the Ag series spherical samples
4. Conclusions
In this work, we studied the effects of zeolite treatment and activation on the physical and chemical properties of activated carbon spherical samples. From the XRD patterns of the prepared spherical samples, it can be seen an AZP (4, 6, 8), zeolite skeleton is basically retained, which is in good agreement with the orthorhombic phase of ZSM-5 zeolite, while the typical characteristic peaks of the main components, SiO2 and Al2O3, were also detected. Photographs of the prepared spherical samples and the SEM results of the prepared composites indicate the important relationship between the amounts of zeolite and AC, with the proper amount of zeolite being 6%. All of the samples had much more iodine adsorption capability after activation than they did before activation; the iodine adsorption capability of AZP (4, 6, 8) was found to decrease with an increasing amount of zeolite and decreased BET surface area, with values as follows: 1194 m2/g, 1011 m2/g, and 982 m2/g, respectively. For the AZP6 series spherical samples prepared with different amounts of PR (10~35%), ignition temperature was conspicuously enhanced to about 589°C. Meanwhile, different amounts of Ag+ treated AZP6 series spherical samples exhibit excellent bactericidal effects for B. Subtilis and S. Epidermis.