1. Introduction
Recently, superparamagnetic iron oxide nanoparticles (SPIONs) have been the focus of extensive attention because such nanoparticles can be easily manipulated or transported by applying an external magnetic field gradient. 1) SPIONs hold enormous potential for applications in nanomedicine such as drug delivery,2) hyperthermia,3) and as magnetic resonance (MR) contrast agents4,5) due to their outstanding magnetic and physiochemical properties, passive/active target specificity, biodegradability, and biocompatibility.
Various synthetic techniques have been reported to produce SPIONs, including thermal decomposition of organometallic precursors,6,7) co-precipitation,8,9) inert gas condensation, nanoemulsion,10) magnetobacteria,11) or laser pyrolysis.12) The controlled chemical co-precipitation method is a commonly used method to synthesize clinically approved dextran coated iron oxide nanoparticles (Feridex®).13) Co-precipitation of Fe2+ and Fe3+ under alkali conditions is required to prevent the oxidation of Fe2+ and to avoid the phase transition from magnetite to maghemite; therefore, it is quite vital that the reaction should be performed in an inert atmosphere, which can be done by directly flowing an inert gas into the solution during coprecipitation of the metal ions. It is already well reported that magnetite nanoparticles are easily oxidized and changed to maghemite in the presence of oxygen, such as dissolved oxygen dispersed in an aqueous phase or when exposed to air. The process of co-precipitation involves an initial nucleation stage for the formation of a new phase, i.e., a phase transition from liquid to solid, followed by crystal growth as the solutes spontaneously assemble on the surface of the nucleus of particles. Several factors, including pH, reaction time and temperature, and concentration of iron salts, can influence the co-precipitation process such that these reaction parameters can be altered in order to tune the properties of the resulting SPIONs. The synthesis of SPIONs by the thermolysis of an iron oleate (FeOl) complex has attracted significant attention compared to other methods, such as hydrothermal and other organic-phase syntheses, due to its applicability to mass production and use of inexpensive and nontoxic metal salts such as iron (III) chloride (FeCl3) and sodium oleate as reactants. Moreover, thermal decomposition of the FeOl complex can produce nanoparticles with different shapes, such as spherical, cubic, and frame shaped with different sizes ranging from 8.5 to 25 nm. FeOl prepared by the conventional method is in a hydrate form and this synthesis process, including purification, is quite complicated and time consuming. Particularly, the FeOl is quite problematic to control because it is a sticky, viscous solid. This tar-like state makes it difficult to accurately weigh out stoichiometric compositions of precursors, which is one of the most important parameters to control in order to tune the diameter and homogeneity of the particles.
Here, we report on a simple and eco-friendly technique to produce non-hydrate FeOl complexes for the formation of highly crystalline fatty acid modified SPIONs by thermolysis of FeOl complexes in a non-coordinating solvent. FeOl complexes were prepared by direct coordination of iron ions and carboxylic acid; thus, we could control the stoichiometric compositions of precursors by manipulating the molar ratio of fatty acid and metal ions. To identify the formation of FeOl complexes and SPIONs by heating, thermal analysis techniques were used to interpret the possible thermal decomposition mechanism for non-hydrate FeOl complexes. The thermolysis of FeOl starts with the splitting of a coordinating bond between a metal ion and an organic molecule, and the thermolysis temperature can serve as an indicator of the strength of the chemical bond. The thermolysis of FeOl involves the liberation of metal ions from carboxylic acid. As a result, monitoring the thermal properties of FeOl will provide some insight into the mechanism of thermal decomposition of metal-fatty acid complexes.
2. Experimental Procedure
2.1. Materials
Iron nitrate nonahydrate (Fe(NO3)3·9H2O), cis-9-octadecenoic acid (OA, CH3(CH2)7CH=CH(CH2)7COOH, 90.0%), hexane (99.0), ethanol (95%), and 1-octadecene (CH3(CH2)15CH =CH2, ODE, 90.0%) were obtained from Sigma-Aldrich.
2.2. Synthesis
Non-hydrated FeOl was prepared by direct reaction of a fatty acid with a metal salt at elevated temperatures. Briefly, 0.4 g of Fe(NO3)3·9H2O was firstly mixed with a variable amount of OA in a three-neck flask. A proportional-integral-derivative (PID) controller was used to control the heating mantle. The temperature was increased to 110°C to eliminate any physically adsorbed water and byproduct, HNO3. During the heating, the solution colour changed from yellow to reddish brown. The temperature of the solution was decreased to 25°C and samples were collected for analysis.
Next, 1 mmol (0.4 g) of Fe(NO3)3·9H2O and 1-5 mmol of OA were dissolved in 5 mL of ODE. When the amount of OA is increased, the non-hydrated FeOl complexes tend to form a more tacky liquid; FeOl-14 and FeOl-15 are in a viscous, reddish brown phase. The solution was heated to 110°C for 2 h to remove the physically adsorbed water and nitric acid. After 30 min, an air condenser was connected for refluxing. The reaction mixture was then heated to 320°C for 30 min with a heating rate of 3.3°C/min under stirring. The color of the reactant changed from reddish brown to black. The fatty acid-coated SPIONs were dissolved in 10 mL hexane, precipitated by adding 20 mL ethanol, and centrifuged at 10,000 rpm for 20 min. The washing procedure was repeated 10 times and the fatty acid-coated SPIONs were re-dispersed in 10 mL hexane, forming an extremely stable ferrofluid. The resulting fatty acid-coated SPIONs are denoted as N1X53 (X = 1, 2, 3, 4, 5). For example, N1253 implies fatty acid-coated SPIONs synthesized with 1 mmol Fe(NO3)3·9H2O and 2 mmol OA in 5 mL of ODE after reacting for 30 min at 320°C.
2.3. Characterization
The size and morphology of the nanoparticles were examined using two transmission electron microscopes, model JEOL 2100F (200 kV) and 1230 (120 kV, Japan). The physical sizes of the iron oxide cores were measured on the JEOL 2100F (200 kV), while both core and shell structures were observed using the JEOL 1230 (120 kV). The average sizes were calculated by fitting the experimental results to a lognormal distribution function:14
where Dp is the mean of the diameters of the particles and ωP is the standard deviation around InD0p.
The mean diameter (Dp) can be written as:
And the standard deviation (σp) of the mean diameter can be written as:
Fourier transform infrared (FTIR) spectra were recorded using an Alpha FTIR spectrometer equipped with Diamond ATR (Bruker Optics). The samples were vacuum dried at 50°C for 24 h and crushed into fine powder for measurement. Spectra were recorded with a resolution of 1 cm−1 in the range of 500-4,000 cm−1.
Thermal gravimetric analysis (TGA), differential scanning calorimetry (DSC), and differential thermal analysis (DTA) analysis were carried out using a Q600 simultaneous DSC-DTA-TGA system from TA Instruments.
3. Results and Discussion
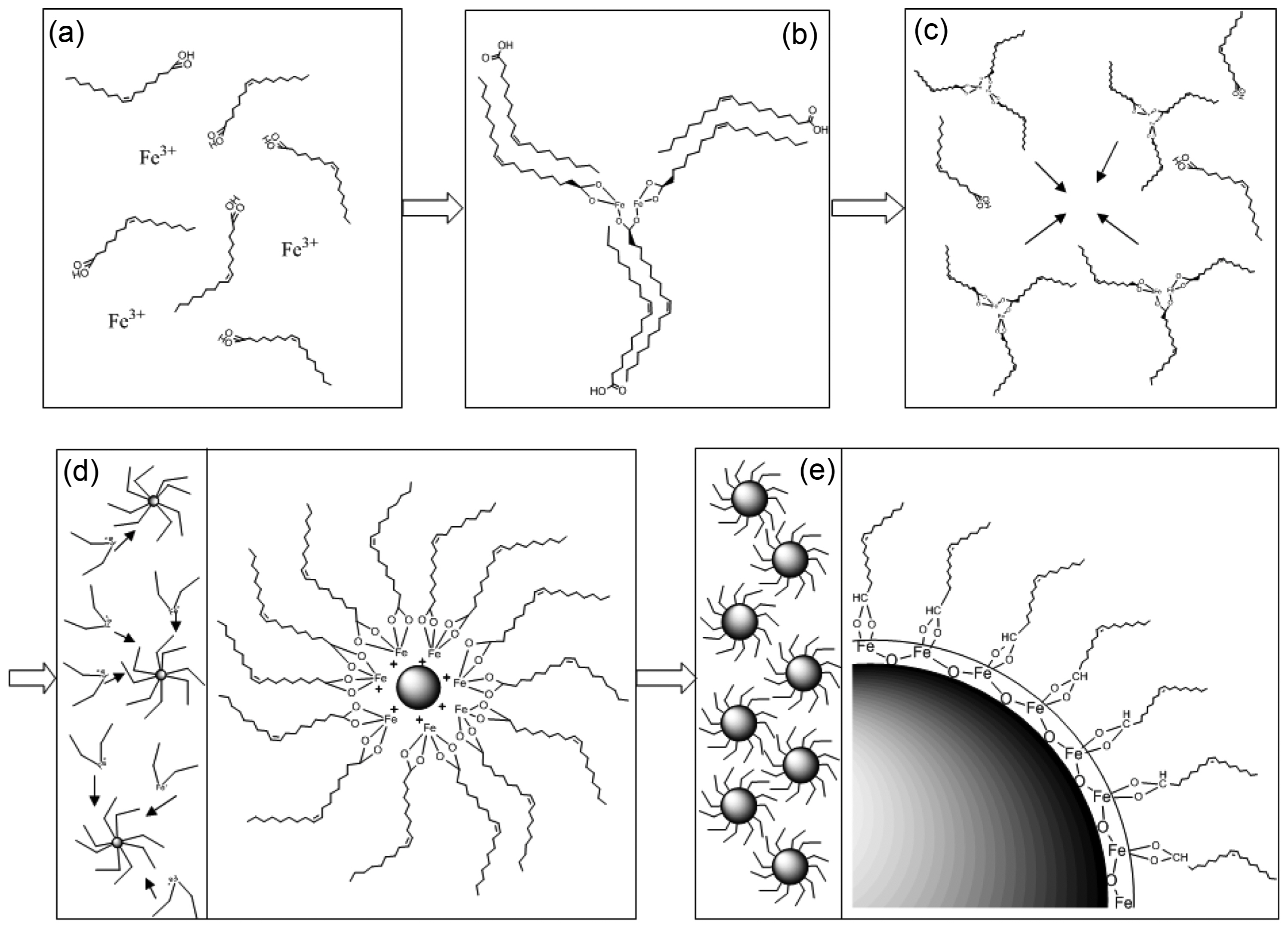
Figure 1 shows a schematic illustration of the fabrication process and thermal decomposition of iron(III) oleate complexes leading to monodispersed SPIONs: (a) dissolution of iron(III) nitrate salts in oleic acid; (b) formation of iron(III) oleate complexes at 120°C and unreacted OA dimerizing with oleate ligands via hydrophobic interactions; (c) breakdown of the hydrophobic interactions between free OA and oleate ligands in the FeOl structure at ~ 180°C; (d) decomposition of oleate ligands from the FeOl and formation of metastable nuclei at 200 ~ 250°C; (e) complete removal of the residual oleate molecules from FeOl leading to growth of SPIONs above 320°C. The structural influences of different concentrations of OA were studied for the coordination of non-hydrated FeOl. Fig. 2 presents FTIR spectra of non-hydrated FeOl synthesized by varying the ratio of Fe(NO3)3 and OA from 1/1 to 1/5. In the spectrum of pure OA, two bands appeared at 2,925 and 2,852 cm−1 which are due to the symmetrical and asymmetrical-CH2 stretching modes. There is a small peak at 3,012 cm−1 that can be assigned to ν(C-H) of the C-H bond that is adjacent to the cis C=C bond.15) A very shallow shoulder appeared at 2,960 cm−1 and was assigned to the asymmetrical-CH3 vibration mode.15) The peaks at 1,456 and 934 cm−1 were assigned to O-H in-plane and out-of-plane bending, respectively.16) The intense band observed at 1,709 cm−1 was attributed to the ν(C=O) of the carboxyl group.16,17)
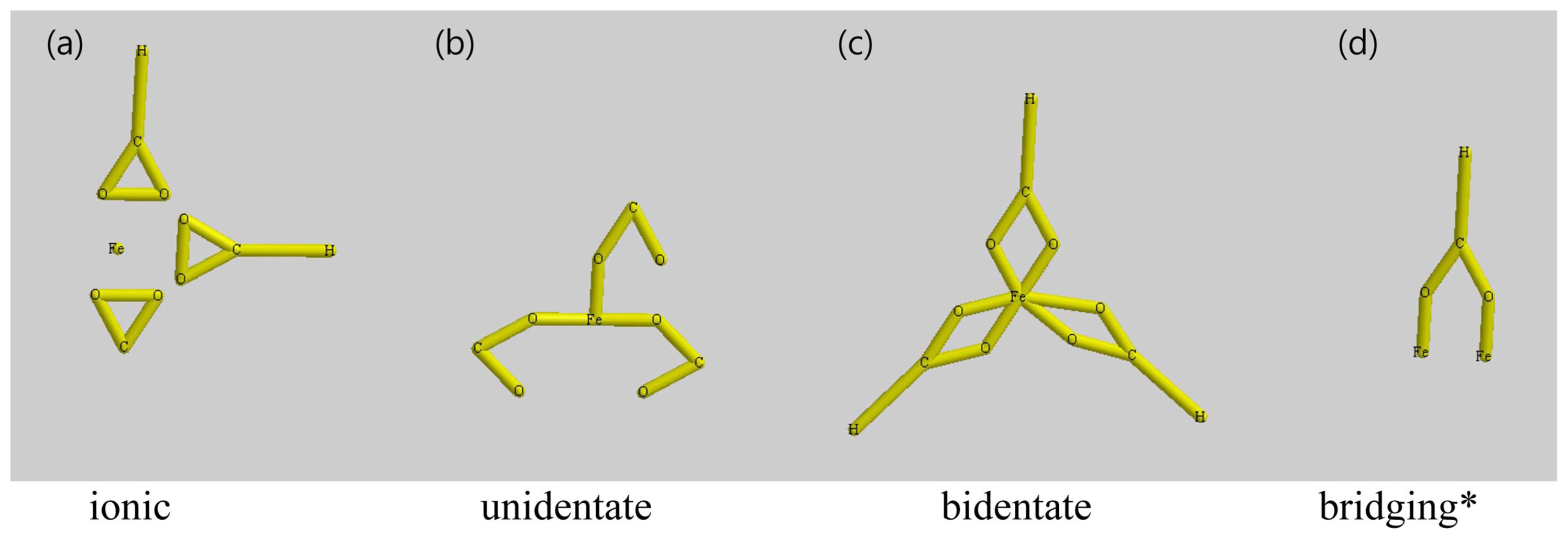
Compared to pure OA, several different peaks appeared in the range of 1,650-1,500 cm−1 in all the case of non-hydrated FeOl due to freshly formed metal carboxylate bonds. A sharp peak at 1,434 cm−1 was also attributed to the vibration of metal carboxylate bonds. Peaks at 1,588, 1,553, and 1,523 cm−1 were attributed to νa(COO−) of FeOl-13, 14, and 15, among which the peak at 1,553 cm−1 had the strongest intensity. However, in FeOl-11 and 12, the peak was too weak to identify. The amplitude of the characteristic peak of unreacted OA at 1,709 cm−1 increased as the molar ratio of Fe(III)/OA was increased, with FeOl-15 showing the highest intensity of the 1,709 cm−1 band, which can be explained by an increasing amount of unreacted OA. In FeOl-11 and 12, distinguished peaks appear at 1,551 cm−1 and 1,434 cm−1, which can be assigned to asymmetrical and symmetrical carboxylate vibrations, respectively, giving a Δ = 117 cm−1, indicating that only the bidentate mode exists in these FeOl complexes. With increased OA concentrations, other asymmetric vibration bands of metal carboxylate appeared at 1,588 cm−1 and 1,523 cm−1, resulting in novel Δ = 154 and 89 cm−1. Therefore, in FeOl-13, 14, and 15, the hydrogen of the carboxylic acid binds to Fe ions in both bridging and bidentate forms.
In the spectrum of FeOl-14, two-CH2 bands (2,925 and 2,852 cm−1) and a-CH3 band (2,960 cm−1) were observed at the same position as in the spectrum of pure OA. However, the peak at 3,012 cm−1 was not observed in the spectrum of FeOl-14, possibly because the cis C=C was converted into trans C=C. Mossoba et al. reviewed FTIR methodologies for detection of trans-fatty acids in which bands at 965-975 cm−1 were also used to differentiate cis and trans substituted fatty acid and esters.18) A new peak appeared at 965 cm−1 in the spectrum of FeOl-14 due to the presence of trans C=C bonds. Apparently, the C=C bond of the OA was converted to a trans-configuration during the coordination reaction.
There is a very strong peak at 1,709 cm−1 in the FTIR spectrum of OA, so it is more sensible to assign this peak to the ν(C=O) stretching mode of free OA. Whereas no 1,709 cm−1 peak was observed in the spectrum of FeOl-14 because unreacted OA was removed during the ethanol wash. The intensity of O-H bond out-of-plane and in-plane bands (1,456 and 934 cm−1 respectively) in the spectrum of FeOl-14 were dramatically reduced and even difficult to distinguish, possibly due to the reduced amount of free OA in the specimen. In the range of 1,650-1,500 cm−1, a number of peaks were attributed to Fe carboxylate bands, as previously reported by Hyeon et al.19) Three bands at 1,588, 1,553, and 1,523 cm−1 are due to asymmetrical carboxylate vibrations, whereas the strong band appearing at 1,436 cm−1 is due to symmetrical carboxylate vibration.20) The chemical coordination of FeOl can be identified based on the location and separation of asymmetrical and symmetrical iron carboxylate peaks, denoted as the splitting amount Δ.17) For Δ > 200 cm−1, a unidentate mode can be expected, for 110 cm−1 < Δ < 200 cm−1 it is a bridging mode, and for Δ < 110 cm−1 it is a bidentate mode. The four coordinating modes between a metal ion and carboxyl groups are schematically illustrated in Fig. 3.
For FeOl-14, the variances between distinctive bands within the ν(COO−) were 152, 117, and 87 cm−1, showing bidentate and bridging modes. Whereas, in FeOl-14, the peaks appearing at 1,553 and 1,523 cm−1 correspond to asymmetrical vibrations, and the strong peak at 1,436 cm−1 can be assigned to a symmetrical vibration.
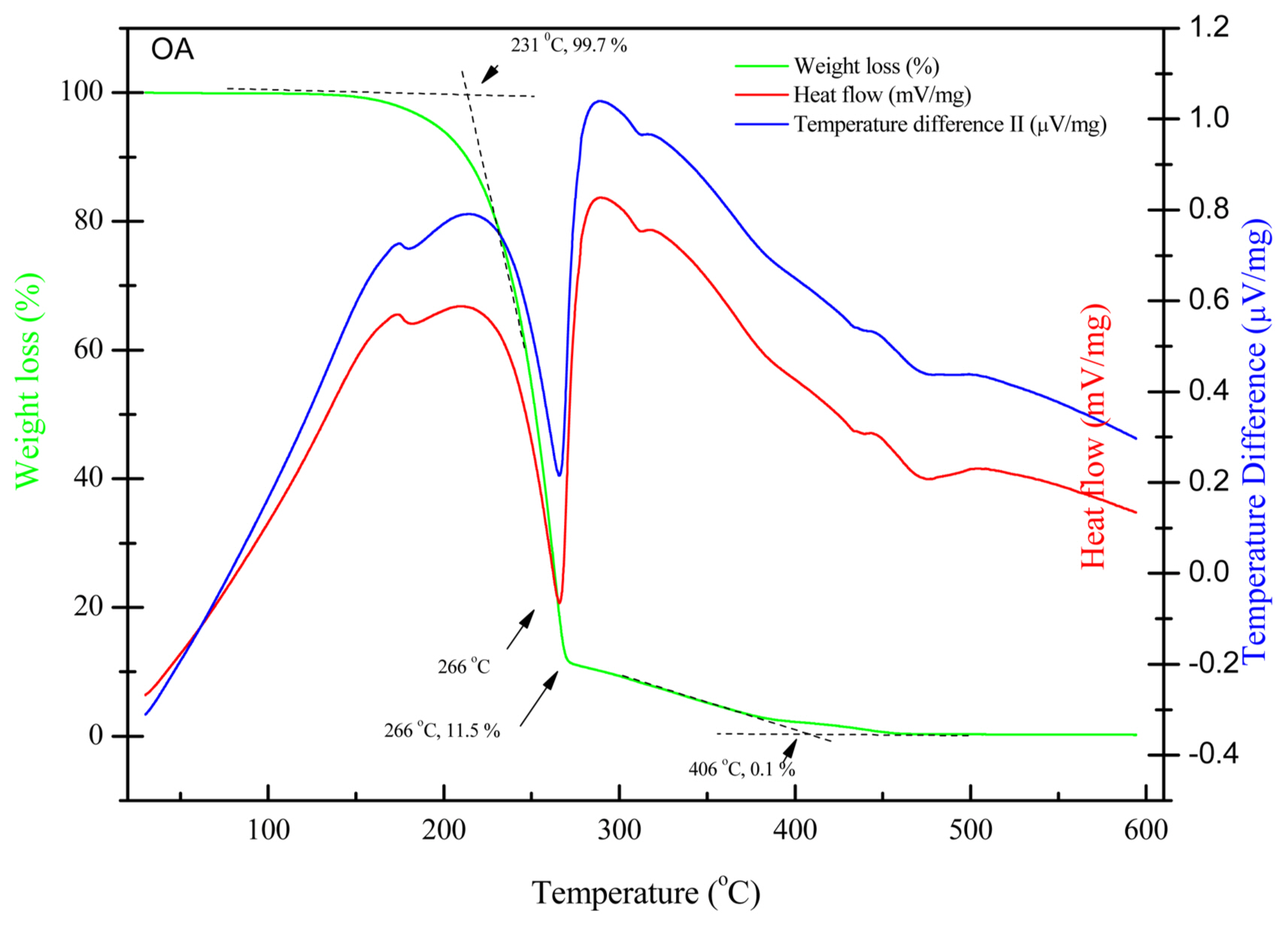
The thermal behavior of OA and non-hydrated FeOl was measured by thermal analysis. Fig. 4 shows the TGA, DSC, and DTA curves for the decomposition of pure OA up to 600°C. The DSC and DTA curves exhibit very similar shapes, since both techniques are concerned with energy changes of the materials undergoing physical and/or chemical changes. The first exothermic transition peak appeared at 174°C without significant weight loss in the TGA curve, possibly due to the dissociation of the dimerized cis-9-octadecenoic acid. There is an eminent exothermic peak at 265°C suggesting thermal decomposition of OA, which is in good agreement with the literature.20,21) Correspondingly, there is a sharp weight loss of 88.2% in this temperature range, the on-set temperature of thermolysis of OA is 231°C, as shown in the TGA curve, and the mass loss decreased to 11.5% at 266°C, indicating that the most of the cis-9-octadecenoic acid has been decomposed to CO, CO2, H2O, H2, ketones, esters, and hydrocarbons with different chain lengths.22) The mass percentage is further decreased to 0.1% at 406°C indicating that the decomposition of OA is complete.
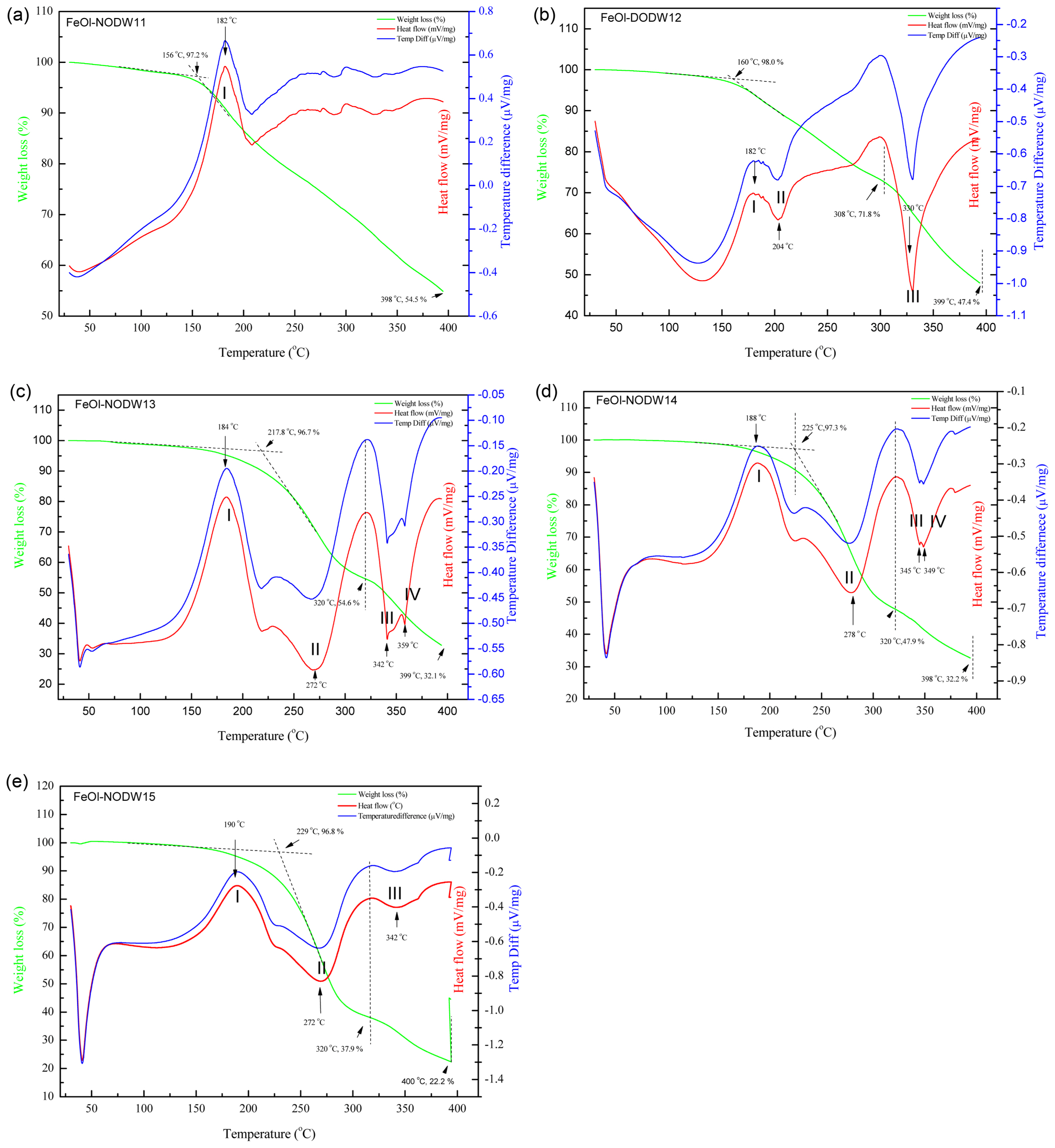
The solid waxy forms of non-hydrated FeOl complexes were studied by thermal analysis to investigate the reaction mechanism for the formation of nanoparticles. Based on FTIR data, non-hydrated FeOl has a combination of excess cis-9-octadecenoic acid and FeOl with different chemical coordination states. Fig. 5 shows the TGA, DSC, and DTA profiles of FeOl-1X (X = 1, 2, 3, 4, 5) from 30°C to 400°C. The first transition of hydrated FeOl samples has been reported at 132°C coming from the chemically absorbed water,17) while no such endothermic peak was observed from any of the non-hydrated FeOl-1X samples. The nonappearance of this peak is a complementary indication supporting the conclusion of the FTIR investigation that no water molecules were incorporated into the non-hydrated FeOl-1X, as shown in Fig. 5(a). The first exothermic transition (I) of non-hydrated FeOl appeared at 179-190°C in all samples, which can be assigned to the breakdown of the hydrophobic interactions between hydrocarbon tails of unreacted OA and oleate ligands in the FeOl structures, because there is no corresponding significant weight loss in the TGA curves. With an increasing molar ratio of cis-9-octadecenoic acid to Fe ions, the exothermic peak was found to be slightly increased, indicating stronger association of oleate ligands and free cis-9-octadecenoic acid. The TGA results show good agreement with the endothermic peak in Fig. 5(b).
In FeOl-11, no significant second transition was observed, indicating that nucleation of FeOl-11 happens over a broad range of temperature resulting in inhomogeneous particle sizes and shapes which is consistent with the TEM results. In FeOl-12, an endothermic peak (II) appeared at 204°C, whereas in FeOl-13, 14, and 15, transition II happened at 272°C, 278°C, and 270°C respectively. The second transition (II) peak which appears around 200-280°C can be attributed to the decomposition of free cis-9-octadecenoic acid or cis-9-octadecenoic acid weakly bonded to Fe atoms in the FeOl structure and the formation of metastable nuclei, because it matches the thermal decomposition of pure cis-9-octadecenoic acid at 266°C. Chemical coordination with Fe atoms stabilizes the oleate molecules and increases the decomposition/dissociation temperature of the oleate ligand. The second exothermic transition peak (II) matches with the second step weight loss between 241°C and 299°C, as shown in the TGA profile of FeOl-14 in Fig. 5(b). The mass loss of the second step was 39.4%, thus the first mass change is due to the elimination of free cis-9-octadecenoic acid and thermal decomposition of one oleate ligand, while the second mass change from 331°C to 400°C is because of the thermal decomposition of the two residual oleate ligands.23)
The endothermic peak above 300°C has previously been attributed to the decomposition of the residual oleate molecules of hydrated FeOl which causes the particle size to increase.24) In FeOl-12, a very deep transition (III) appeared at 330°C, and in FeOl-13, the endothermic peak was split into two peaks positioned at 341°C (III) and 359°C (IV). In FeOl-14, endothermic peaks (III) and (IV) were found at 345°C and 349°C. As the Fe(III)/cis-9-octadecenoic acid molar ratio increases, peak (III) is right-shifted and becomes shallower. Correspondingly, in FeOl-14, the remaining weight loss is 34.6%, which can be considered as the formation of iron oxide nanoparticles. The substantial separation between the second (II) and third (III) thermal transition peaks is strong evidence that nucleation and particle growth occur in different stages. Bronstein et al. attributed the peak in the region of 340°C-380°C to comprehensive thermal dissociation or restructuring of hydrated FeOl.20) Only a shallow endothermic peak (III) was observed at 342°C in FeOl-15, and no third endothermic peak was detected.
Particle size distributions calculated with TEM images of N1153 ~ N1553 synthesized with varying molar ratios of Fe(III)/cis-9-octadecenoic acid are shown in Fig. 6(a)-(e). By varying the molar ratio of Fe(III)/cis-9-octadecenoic acid from 1/1 to 1/5, the diameter of the resulting nanocrystals can be tuned at a fixed reaction temperature (320°C) and time (30 min). Nanocrystalline iron oxide particles with average diameters of 8.4 nm, 11.1 nm, 12.3 nm, 15.0 nm, and 10.0 nm were fabricated in ODE. The particle size could be increased by increasing the ratio of Fe(III)/cis-9-octadecenoic acid from 1/1 to 1/4. Interestingly the particle size starts to decrease when the molar ratio is further raised to 1/5. During particle formation, a lower amount of carboxylic acid compared to metal results in more asymmetrical size distribution and morphology. The particle diameter continuously increased when the molar ratio of cis-9-octadecenoic acid was increased to 4, but the particle diameter started to decrease afterward. When free cis-9-octadecenoic acid exists during particle formation, the free carboxylate acids will be attached on the surface of the nucleated particles and react again with iron ions as a reverse reaction. As a result, iron (III) oleate starts to leach out into the solution.
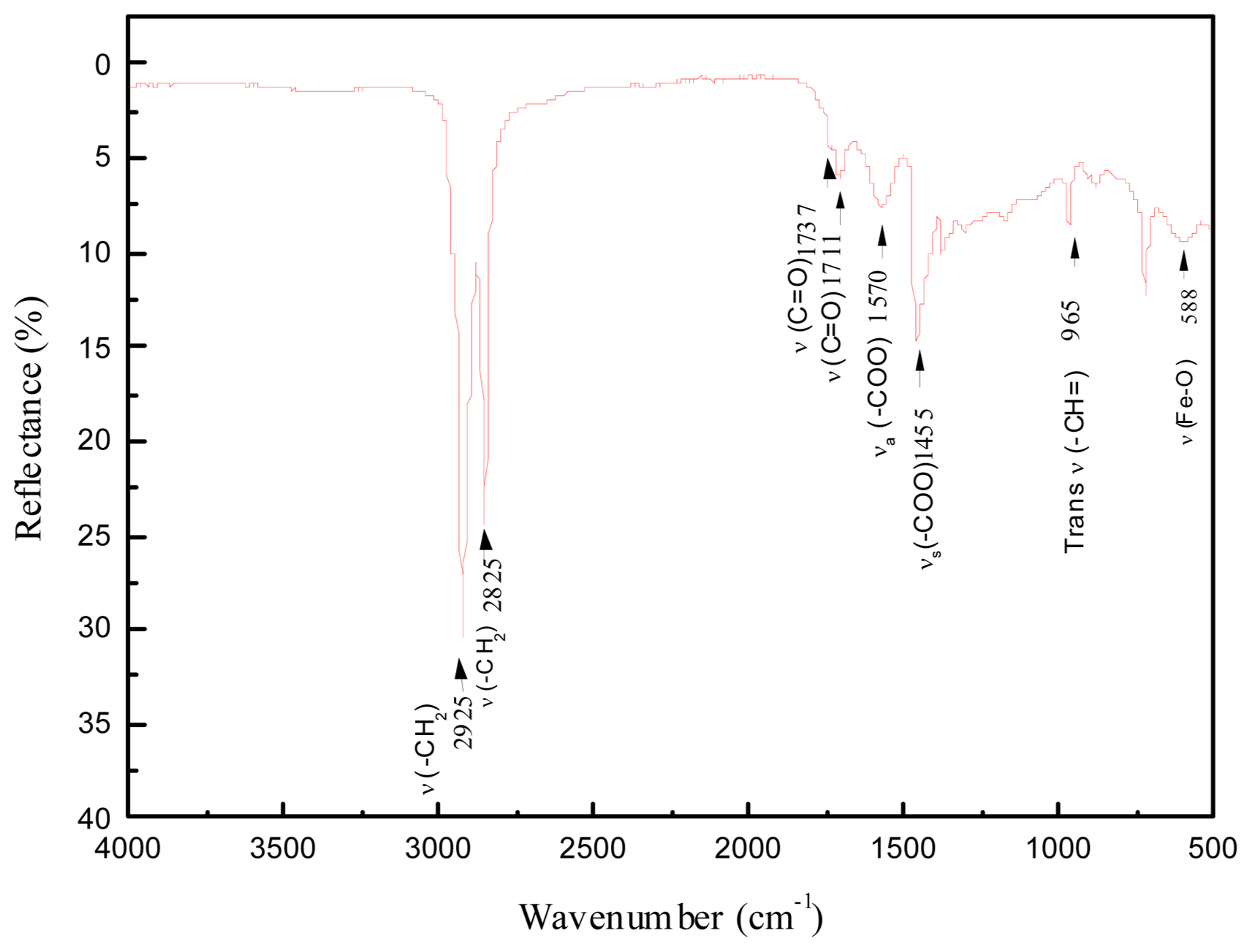
FTIR spectra of N1553 from a wavenumber of 4,000 cm−1 to 500 cm−1 are shown in Fig. 7. Two strong peaks at 2,925 and 2,852 cm−1 can be assigned to symmetrical and asymmetrical-CH2 stretching.25,26) Two bands appearing at 1,737 cm−1 and 1,711 cm−1 can be attributed to C=O stretching vibrations of monomer and dimer cis-9-octadecenoic acid, respectively.27) No significant stretching of C=C from cis-9-octadecenoic acid could be distinguished at 1,656 cm−1, possibly due to interference from the 1,711 cm−1 peak.28) The peak at 1,570 cm−1 was attributed to asymmetrical vibration of carboxylate. The 1,455 cm−1 can be attributed to the symmetrical vibration of carboxylate.27,29) Hence based on the classification of coordination modes by determining the Δ value (Δ = 115 cm−1, in this case), we conclude that cis-9-octadecenoic acid binds to the surface of iron oxide in a bridging mode. A weak peak was detected at 588 cm−1 due to Fe-O bonding; nevertheless, the intensity of this peak is quite low and difficult to distinguish, probably because the surfaces of the particles are fully coated with cis-9-octadecenoic acid.
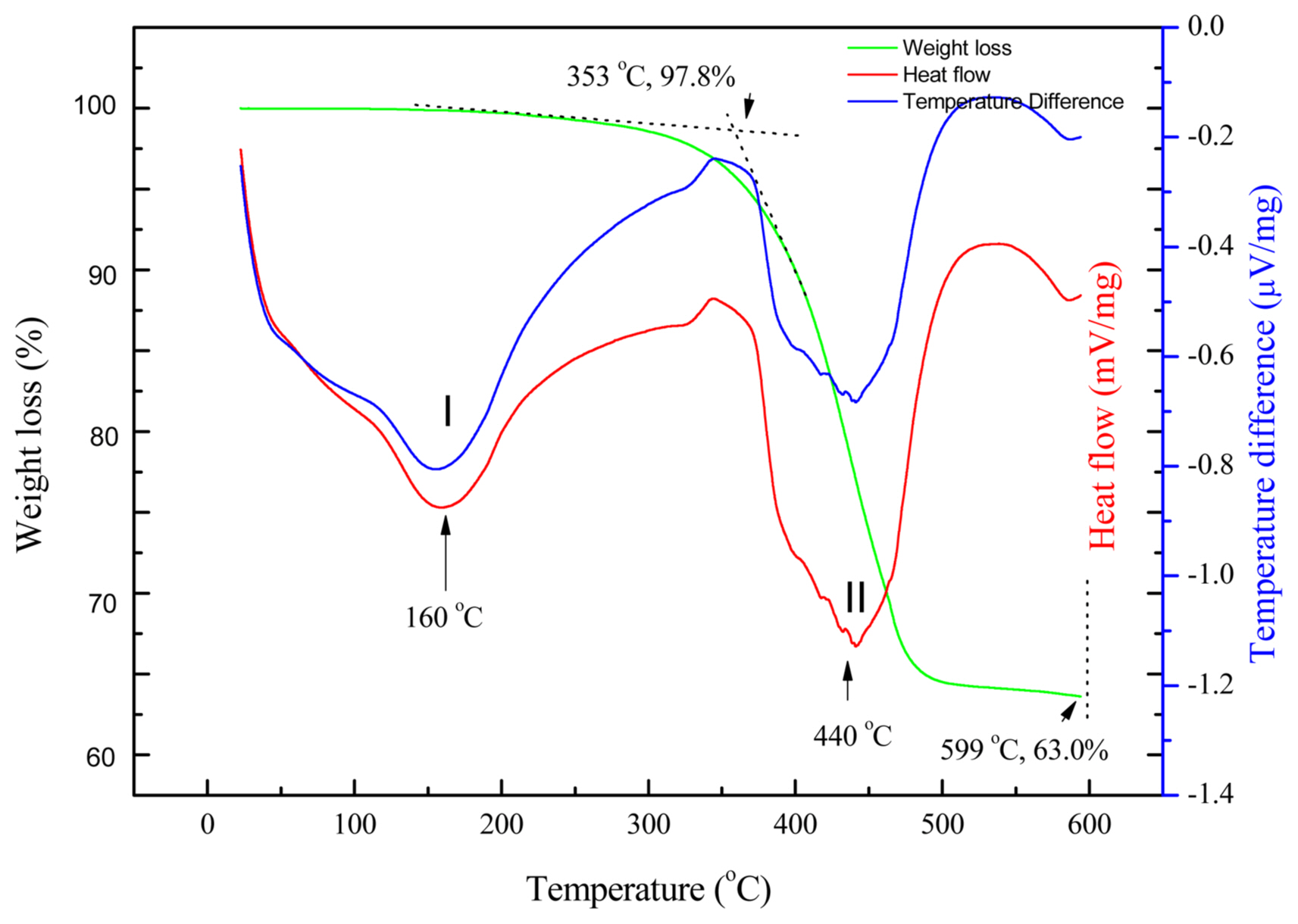
Figure 8 shows the TGA, DSC, and DTA profiles of N1553 between 30°C and 600°C with a heating rate of 10°C/min. The first endothermic transition (I) started around 100°C and the endothermic peak appeared at 160°C in both the DSC and DTA profiles. Correspondingly, the weight loss over the temperature range from 100°C to 300°C is ~ 2.2 %, which is possibly caused by the evaporation of residual ethanol from the wash cycles or absorbed water. The second endothermic transition peak (II) appeared at 440°C in the DSC and DTA curves, corresponding to a major weight loss of 34.8% in the TGA profile, which is attributed to the adsorption and decomposition of cis-9-octadecenoic acid molecules. The mass of the remaining inorganic materials was determined to be 63.0% at 600°C. Therefore, the observed mass ratio of surfactant cis-9-octadecenoic acid molecules to the inorganic cores (Ro) can be calculated:
Where mII is the weight loss percentage of second transition and mr is the remaining weight percentage at 600°C.
According to the TGA profile shown in Fig. 8,
To compare the observed mass and the theoretical value of the mass ratio of cis-9-octadecenoic acid molecules to the inorganic cores, we calculated the mass ratio of cis-9-octadecenoic acid molecules that absorbed on one single nanoparticle and that nanoparticle itself (R):
Where ms is the mass of all the surfactant cis-9-octadecenoic acid absorbed on a single nanoparticle and mNP is the mass of the nanoparticle.
Assuming 100% coverage of the nanoparticle surface, we can calculate the number of the cis-9-octadecenoic acid molecules absorbed on the surface of a nanoparticle (ns):
Where ANP is the surface area of the nanoparticle, As is the molecular area of the carboxylate group of cis-9-octadecenoic acid on the iron oxide surface, and r is the radius of the given nanoparticle.
Hence, the mass of all the surfactant cis-9-octadecenoic acid absorbed on single nanoparticle (ms) can be calculated:
Where Mw is the molecular weight of a cis-9-octadecenoic acid molecule and NA is Avogadro’s number.
And the mass of a single iron oxide nanoparticle (mNP) can be calculated:
Where ρ is the density of iron oxide.
Therefore, we can calculate the mass ratio of surface absorbed cis-9-octadecenoic acid molecules and the iron oxide core of an individual nanoparticle of an average diameter (d) of 10.0 nm:
To simply the whole equation:
Klokkenburg et al. reported a density of 3.5 molecules per nm2 on the iron oxide surface, which corresponds to the area of the carboxylate head group of cis-9-octadecenoic acid (0.28 nm2).30) Rogan et al. estimated the molecular area of the carboxylate group of cis-9-octadecenoic acid on several mineral surfaces to be ~ 20 Å2.31) Yoshida et al. studied several lipid monolayers at the water-oil interface and reported the head group of stearic acid, which has a structure closely related to that of cis-9-octadecenoic acid, occupied an area of 22.5 Å2 at the interface.32) In our case, we assumed the molecular area of the carboxylate group of cis-9-octadecenoic acid absorbed on the iron oxide surface to be As= 0.25 nm2 (25 Å2) and 100% surfactant coverage of the monolayer of surfactant on the surface of the nanoparticles.
Therefore, the theoretical value of the mass ratio of surface absorbed surfactant to iron oxide core was determined to be ~ 0.215
Surprisingly, the observed value (Ro = 0.552) of the mass ratio of surfactant cis-9-octadecenoic acid to inorganic oxide nanoparticle is two times greater than the calculated value (R = 0.215). Zboril et al. proposed two possible chemical reactions between palmitic acid and magnetic nanoparticles, in which the carboxylate head of the inner layer attached to the surface of the magnetic particle and the outer layer palmitic acid interacted with the inner layer surfactant by hydrophobic interactions.33) Klokkenburg et al. reported two endothermic transitions at 260°C and 380°C, respectively. He postulated that there were two binding modes of cis-9-octadecenoic acid to the iron oxide surface on the different crystal facets of the iron oxide nanocrystals; there remained a single layer of surfactant absorbed on the surface of the particle with less than 90% coverage.30) As only the second endothermic transition that was correlated with the major weight loss of 34.8% was assigned to desorption and decomposition of cis-9-octadecenoic acid molecules, it is sensible to conclude that cis-9-octadecenoic acid molecules bind to the surface of iron oxide nanoparticles in one coordination mode. As shown previously in the FTIR spectra of N1553, cis-9-octadecenoic acid binds to the surface of iron oxide nanoparticles only in a bridging mode, which is consistent with the TEM and TGA/DSC/DTA analysis. FTIR data also showed an intense peak at 1,711 cm−1, which is usually attributed to the stretching of C=O in dimer cis-9-octadecenoic acid. Therefore, together with FTIR, TEM and thermal analysis of N1553, we conclude that instead of forming a surfactant monolayer with full coverage on the surface of the iron oxide nanoparticles, it is possible that cis-9-octadecenoic acid molecules formed a “quasi-double-layer” on the surface of iron oxide. In this case, the inner layer of cis-9-octadecenoic acid will bind to the iron oxide via bridging coordination, and the rest of the cis-9-octadecenoic acid molecules assemble into the outer layer by forming dimer cis-9-octadecenoic acid via hydrophobic interactions between the hydrocarbon tails of the cis-9-octadecenoic acid molecules.
4. Conclusions
In this work, we developed a simple and eco-friendly technique to produce non-hydrate FeOl complexes which were used in a one-pot reaction to prepare highly monodispersed SPIONs with tunable size. Potential coordination modes of intermediate non-hydrated FeOl complexes were proposed based on thermal analysis and FTIR data. As shown in the FTIR spectra of SPIONs, cis-9-octadecenoic acid binds on the surface of SPIONs in a bridging mode only, which is consistent with the TEM and TGA/DSC/DTA analysis. We concluded that instead of forming a surfactant monolayer with full coverage on the surface of the SPIONs, it is possible that OA molecules form a “quasi-double-layer” on the surface of SPIONs. Nanocrystalline SPIONs with average diameters 8.4 nm, 11.1 nm, 12.3 nm, 15.0 nm, and 10.0 nm were fabricated in ODE. The particle size could be increased by increasing the ratio of Fe(III)/OA from 1/1 to 1/4, but was found to decrease after further increasing the ratio of Fe(III)/OA to 1/5.