1. Introduction
Commercial glasses, which are used mainly in kitchens and laboratories, require a low coefficient of thermal expansion, good thermal shock resistance, high chemical durability and electrical resistivity, etc. Generally, borosilicate glass satisfies these requirements; there are several commercial borosilicate glasses such as Pyrex® and Duran®. However, the melting points of borosilicate glasses are very high, exceeding 1500°C, resulting in high energy consumption during production. In order to solve this problem, phosphate and borate glasses have been extensively studied. Phosphate glasses, which have a high coefficient of thermal expansion, low melting and softening temperatures, and high electrical conductivity, and which respond to specific types of light, have gained considerable attention over the last two to four years in various fields.1) However, the poor chemical durability of phosphate glasses makes them unsuitable for many commercial applications; to overcome the poor durability, various studies on the dissolution behavior of phosphate glasses have been performed.2-4) Takebe et al. studied the compositional dependence of chemical durability in meta-phosphate glasses, and reported that the chemical durability of ZnO-P2O5 glasses of water was improved by addition of ZnO.3) In the case of zinc borophosphate glass, the first investigation of the glass-forming regions in the ZnO-B2O3-P2O5 system was reported by Ushakov et al.,5) who reported two glasses-forming regions: one adjacent to the ZnO-P2O5 system and the other placed between Zn metaborate and ZnO-3ZnOP·P2O5 composition. L. Koufelka et al. verified the data on glass formation in the ZnO-B2O3-P2O5 system and characterized the obtained homogeneous glasses using physico-chemical and spectral methods.6)
ZnO is interesting not only for its wide variety of physical and chemical properties but also for its antibacterial activity. The antimicrobial properties of ZnO, without toxicity and environmental effects, have been widely investigated. And, ZnO is an effective and stable photocatalyst in the waste treatment and renewable energy fields. It is also known to have antibacterial properties.7-11) Therefore, stable phosphate glasses have attracted significant interest as functional materials. Research into effective photocatalyst powders that can be obtained using a conventional coating or chemical vapor deposition method has attracted attention recently in studies of photocatalytic and antibacterial materials. However, because attempts to assess the photocatalytic and antibacterial functions require the separation of the powder and the solution, such studies are difficult at general laboratory scale. To overcome this problem, it is necessary to fix the material on a substrate.
In this paper, the antibacterial properties and glass structure of borophosphate containing ZnO were studied. The structural changes in zinc borophosphate glass with added B2O3 and the changes in the thermal properties were confirmed by Thermo Mechanical Analyzer (TMA) and Fourier Transform Infrared spectroscopy (FTIR) analysis. Structural analysis was carried out to investigate the effects of B2O3 addition on the chemical durability and CTE changes. In addition, the effect of the Zn2+ content on the antibacterial properties was investigated through X-Ray Photoelectron Spectroscopy (XPS); an antibacterial test with Escherichia coli (E. coli), following the Japanese Industrial Standard JIS Z 2801, showed the relationship between E. coli death rate and Zn2+ content in the glasses.
2. Experimental Procedure
ZnO (Junsei, CP, Japan), B2O3 (Junsei, CP, Japan), and NH4H2PO4 (Junsei, EP, Japan) were used as the starting materials. As shown in Table 1, five glasses with high ZnO content were fabricated. For each composition, 30 g of powder was placed in an alumina crucible and melted in an electric furnace at 1000°C for 1 h. After melting, the sample was poured into a stainless-steel mold and quenched. The quenched specimens were annealed for 1 h at near their glass transition temperature (Tg: 480°C) and cooled slowly. Vitrification of the manufactured glass was confirmed by X-Ray Diffraction (XRD, Rigaku Corporation, Cu, 30 kV, 15 mA) analysis. The glass transition temperature and coefficient of thermal expansion were measured by Thermo Mechanical Analyzer (Q-400 TMA, T.A. Co, USA). FTIR spectroscopy (PerkinElmer, Spectrum GX) with KBr method used to investigate the structure of each glass composition. The investigated sample was dried in a dryer at 100°C and tested after powder milling (particle size of 44 μm or less). KBr powder was mixed at a ratio of 1 : 1200. XPS was performed to determine the Zn state and the structure of the glass. XPS spectra were measured using an ESCALAB XPS and Theta Probe XPS system under monochromatic Al Kα (hv = 1486.6 eV) radiation. An analytical area 400 μm in diameter was used and the results were calibrated using a reference C 1s line (284.6 eV).
In order to analyze the antibacterial properties of the glasses, a Japanese Industrial Standard (JIS) Z 2801 standard test was conducted.12) This test measures that the visible number of bacteria or fungi on a test piece after 1.0 × 107 cells are cultured for 24 h at a relative humidity of > 90% at 35°C. The test was conducted 20 times for reproducibility.
The number of viable bacteria was calculated by the following equation.
where N is the number of viable cells of bacteria, C is the average number of colonies in the two petri dishes adopted, D is the dilution ratio, and V is the volume (ml) of SCDLP broth used for washing out the bacteria.
Antibacterial activity was calculated by the following equation.
where R is the value of the antimicrobial activity, A is the average of the number of viable cells of bacteria after 24 h inoculation of the treated test piece, and B is the average of the number of viable cells of bacteria on the untreated test piece after 24 h. Conversion of antimicrobial activity values can be classified as follows: i) ~ 1.99 = −, ii) 2.00 ~ 2.99 = 99.0%, iii) 3.00 ~ 3.99 = 99.9%, iv) 4.00 ~ = 99.99%.
3. Results and Discussion
3.1. Structure analysis
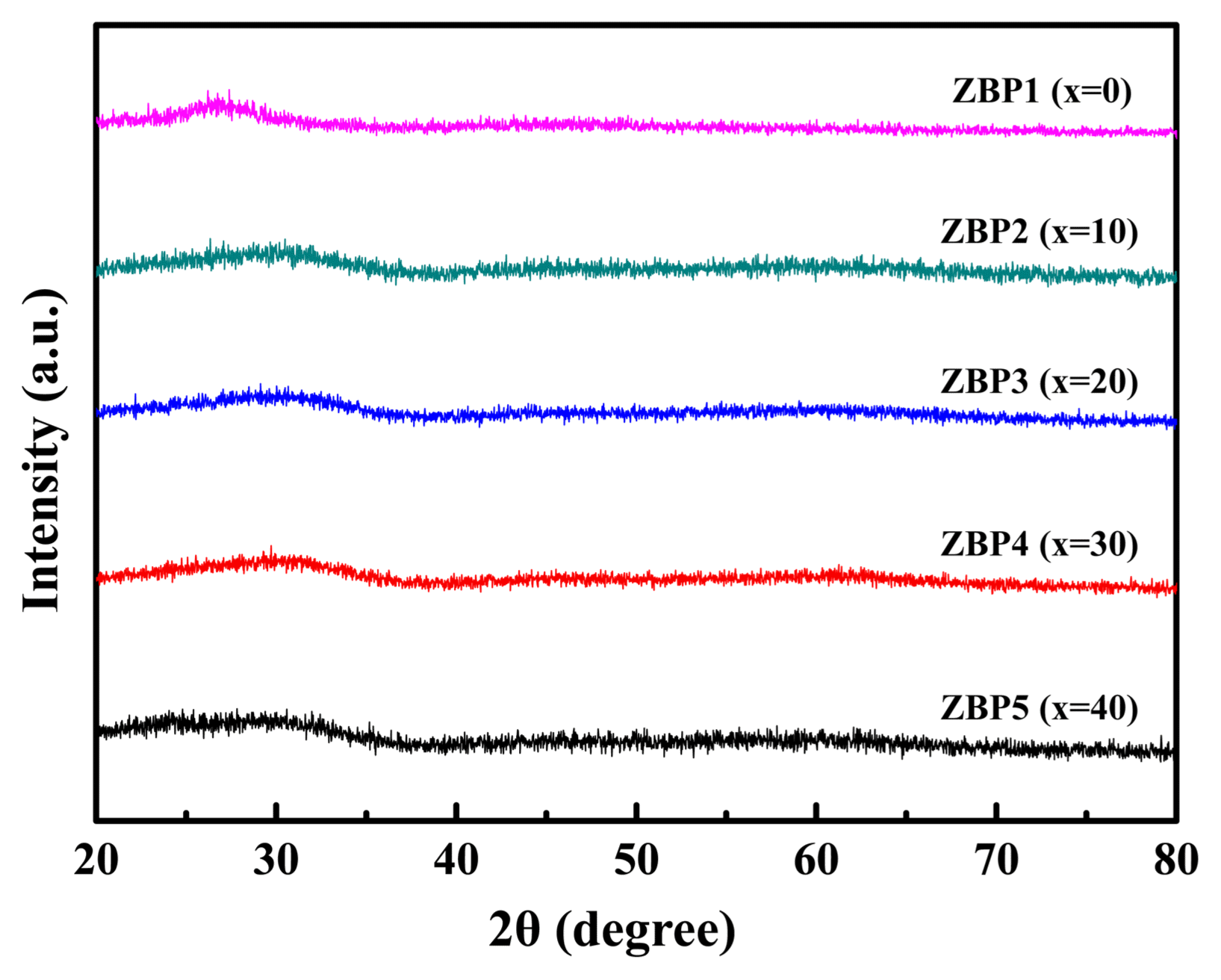
Figure 1 shows the XRD patterns of the (65-x)ZnO-xB2O3-35P2O5 glasses. Patterns are only the characteristic halo pattern of amorphous material at a low diffraction angle (near 2θ −20° - 30°); this clear ups up the idea that there is no crystal effect in this study.
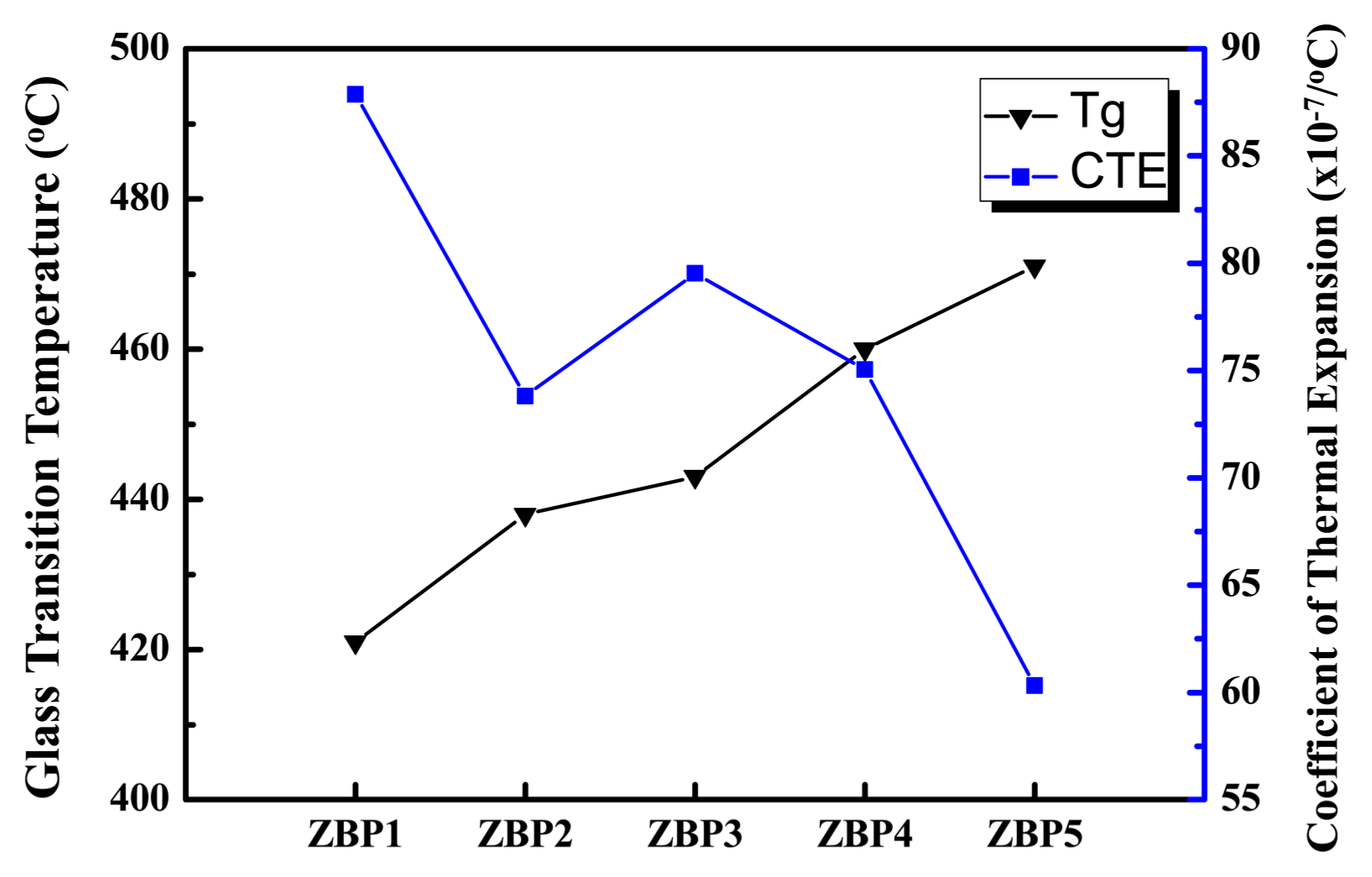
Table 2 and Fig. 2 show the glass transition temperature, Tg, and the coefficient of thermal expansion, C.T.E., in the vitrification zone. The experimental results show that the glass transition temperature increases and the coefficient of thermal expansion decreases with increasing B2O3 content. Brow et al. reported that there are forms of BO3 with three-coordinated boron atoms and BO4 with four-coordinated boron atoms in borate glasses; their mutual participation in the structure of borophosphate glasses was investigated by B NMR analysis.13) Therefore, the incorporation of BO4 units in the chain-like phosphate glass structure results in the enhancement of dimensionality of the structural network from one-dimensional to three-dimensional. Such a cross-linking of the structural network gives glasses with increased Tg; the thermal expansion coefficient generally decreases with increasing content of B2O3. In the case of ZBP3 (20 mol% B2O3), the coefficient of thermal expansion increases because of boron anomalies in which BO3 and BO4 units are mixed. When the B2O3 content exceeds 20 mol%, the bridging oxygens are exhausted and B2O3 tends to occur in the form of planar BO3 units.6)
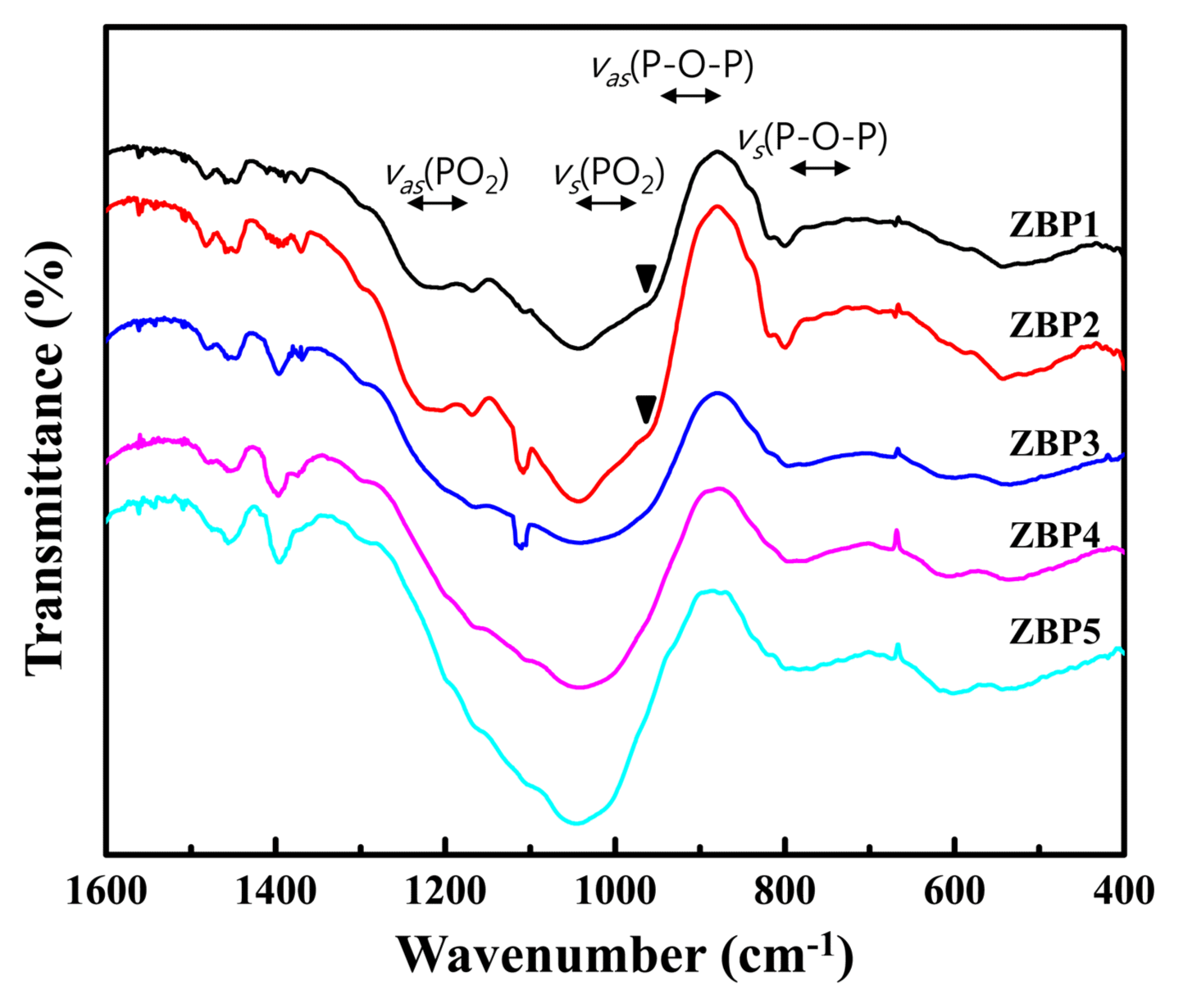
Figure 3 shows the FTIR spectra of the (65-x)ZnO-xB2O3-35P2O5 glasses. Phosphate glasses are composed of network forming, corner-sharing PO4 tetrahedra, in which three corners are shared and a double bond forms between the phosphorus atoms and one of the four oxygen neighbors. The band in the 1200 - 1250 cm−1 range is ascribed to asymmetric stretching vibration vas(PO2) of nonbridging oxygen and another band in the 1000 - 1080 cm−1 range is assigned to the symmetric stretching vibration vs(PO2) of nonbridging oxygen in the phosphate chain. The band in the 900 - 970 cm−1 range belongs to asymmetric vibrations vas(P-O-P) of bridging oxygen and the weaker band at 700 - 800 cm−1 is assigned to symmetric stretching vibration vs(P-O-P) of bridging oxygen in the phosphate chains. The broad band at 500 cm−1 belongs to the bending vibrations of basic structural units of phosphate glasses.14) The vibration at 1000-1200 cm−1 is the result of vibration of the B2O72− ionic group, which indicates that planar BO3 units are changing into tetrahedral BO4. With increasing B2O3 content, the high-frequency bands corresponding to stretching vibrations become broader and less distinct and overlap each other.6) The results for samples ZBP4 and ZBP5 indicate that the rapid increase in the content of planar BO3 units weakens the glass structure because the bridging oxygens are exhausted when B2O3 > 20 mol%. The peak at 970 cm−1, which is shown in the high ZnO content samples, results from a combined effect of Zn-O stretching vibration, [ZnO6] characteristic absorption, and P-O-P asymmetric vibration.15)
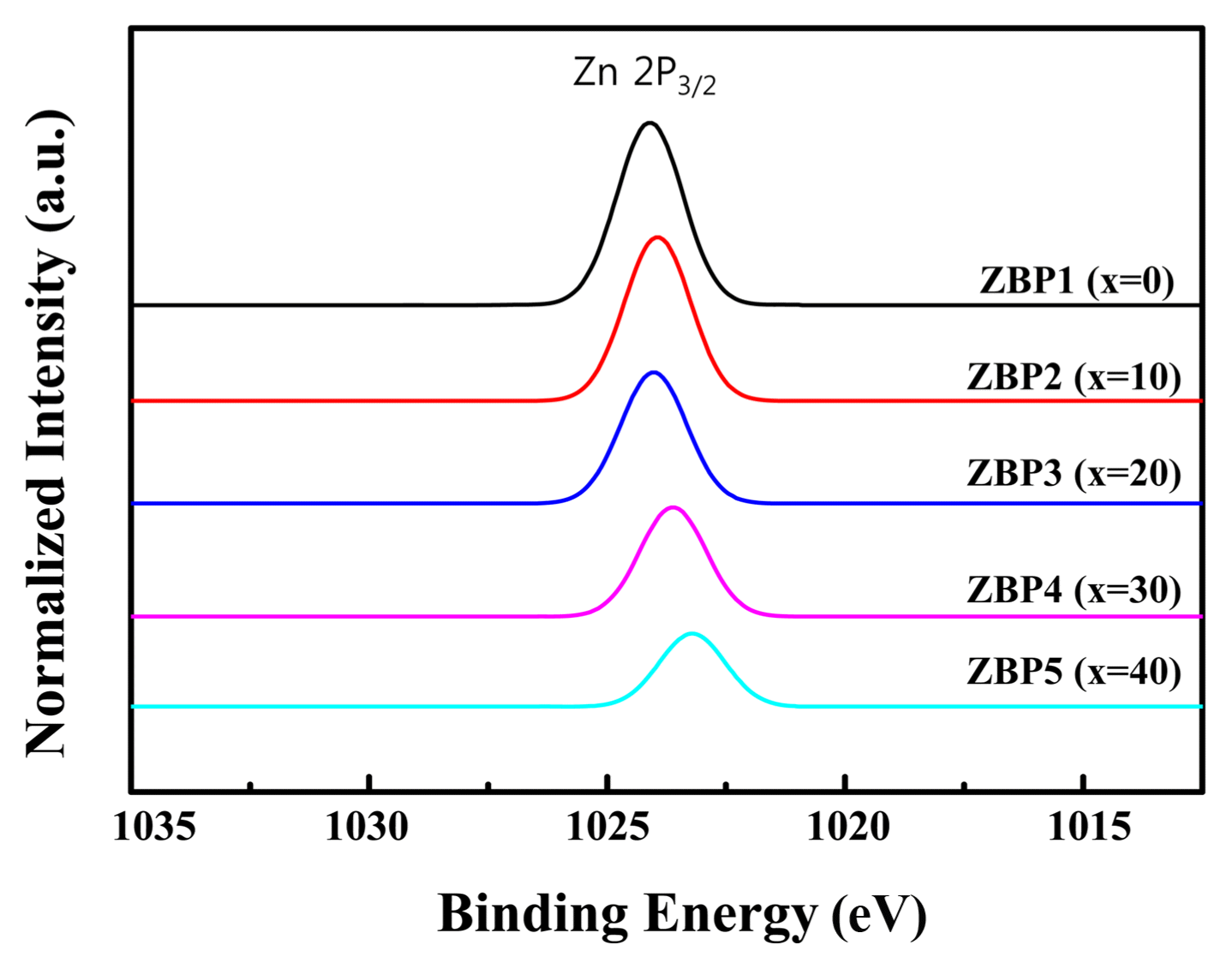
The ZnO ionic state was investigated by XPS (Fig. 4). The peak movement (1023.3 eV to 1024.2 eV) with increasing ZnO showed the same results as in a previous study; the single peak of Zn 2P3/2 show that ZnO exists only in the Zn2+ state.16) The peak intensity can be confirmed for which Zn2+ increases with increasing ZnO content.
3.2. Antibacterial property
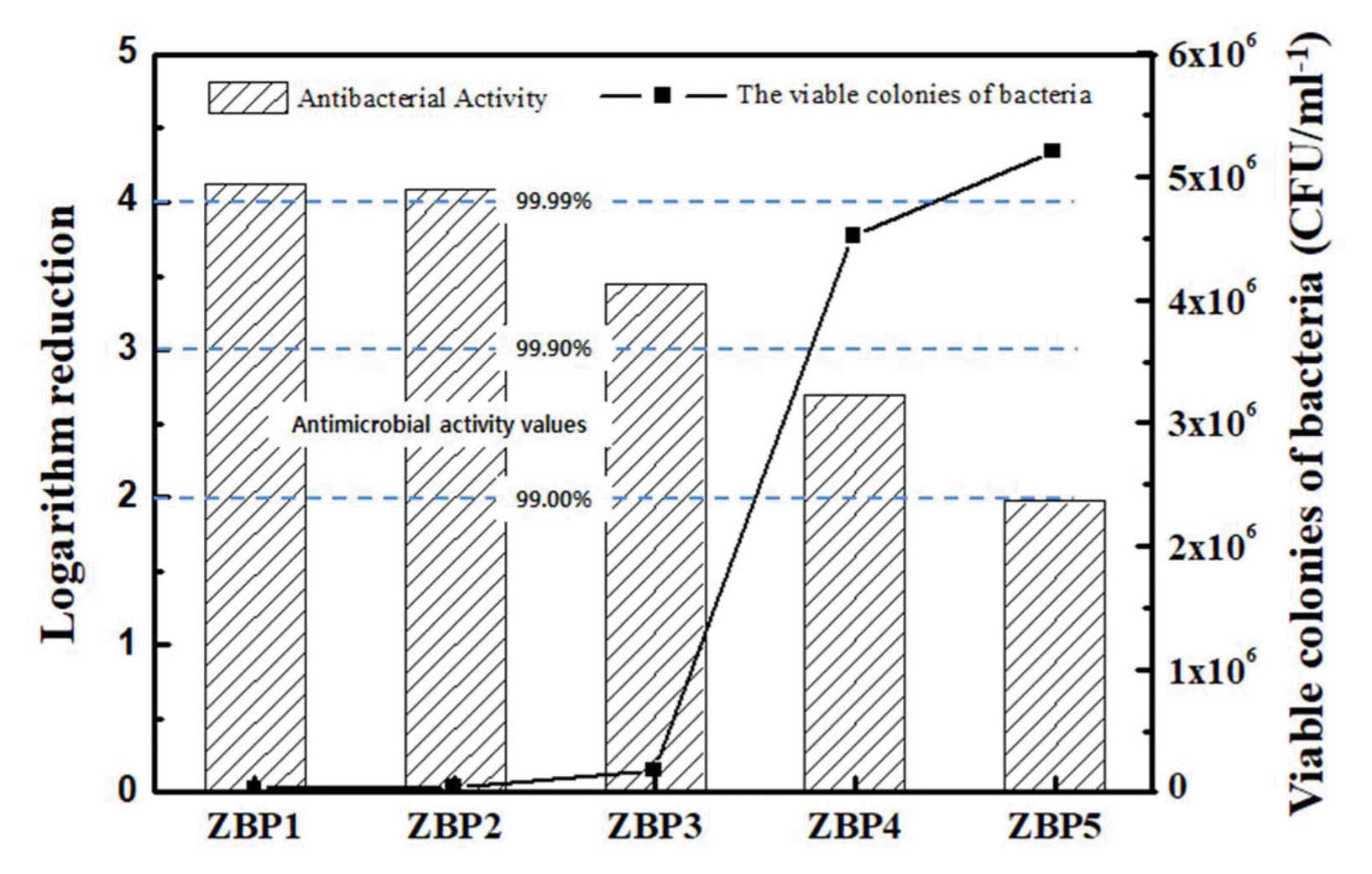
Figure 5 and Table 3 show the rate of decrease of E. coli obtained using the JIS Z 2801 standard test. The E. coli death rate increases with increasing ZnO content of the glass and with ZBP 1 and ZBP 2; the death rate is 99.99% or more, which is superior to those of the other three samples. Padmavathy et al.17) reported that ZnO can be activated with visible and/or ultraviolet light, generating reactive oxygen species (ROS); H2O2 penetrates the membrane, causing oxidative stress and the death of the cell. Xie et al.18) suggested that ZnO has a direct effect on the membrane, modifying its permeability, which increases the ZnO concentration inside the cell, where it induces oxidative stress. Those studies state that Zn2+ directly induces a significant increment in intracellular reactive oxygen species (ROS) due to the Zn2+ interacting with the thiol-groups of the enzymes in charge of the breathing cycle of the bacteria; the result in this study also suggests that the antibacterial mechanism of ZnO is likely the oxidative stress in the E. coli.
4. Conclusions
The glass structure and antibacterial properties of ZnOB2O3-P2O5 glasses were studied. B2O3 in the cross-linking of phosphate glass structure brought an increase in the glass value of Tg and CTE decreased with increasing B2O3 amount. The single peak of Zn 2P3/2, obtained by XPS, showed the ZnO state: Zn2+ increases with increasing ZnO content. The viable colonies of bacteria decreased with increasing ZnO; ZnO-B2O3-P2O5 glass with high ZnO concentration in this work showed over 99.99% antimicrobial activity values.