1. Introduction
The Kramers-Kronig (KK) technique has been used to evaluate the reflection spectra to determine a number of optical parameters of materials.1-5) This may be because this technique is easier to develop and can be applied to scrutinize a wide variety of materials without reference to detailed models. There has been great interest in the KK technique for the evaluation of the IR reflectance spectra of several materials. These spectra are determined by the broadly used Fourier transforms infrared (FTIR) spectroscopy.
Ceramics are common materials used to improve certain physical and mechanical properties of polymers such as the hardness, Young’s modulus, creep resistance and thermal stability.6) Although many studies have been carried out to investigate the effects of ceramics on mechanical properties, optical features as well may change when using ceramic materials in the polymer matrix.7,8) Polymethyl methacrylate (PMMA) is one of the most important acrylates due to its exceptional optical clarity9) and mechanical properties.10) It is possible to change some of the optical constants of polymers in a number of frequencies by using a proper ceramic as a filler. Aluminum oxide, or alumina, is a valuable ceramic material due to its prominent mechanical, thermal, optical, and chemical properties, which provide new opportunities for applications as both bulk and filler.7,11-17) By using alumina as a filler, the physical and mechanical properties of polymers can change, but, first, the surface must be sufficiently modified18) so as to be able to bond suitably to the polymer chains. Sufficient information about the content of the coupling agent necessary for acceptable surface modification is necessary to reach the best grafting of the coupling agent to the surface of the alumina nanoparticles. Grafting density and grafting yield will be calculated by using CHN elemental analysis which is a scientific instrument that can determine the elemental concentrations in a given sample and is used to measure Carbon (C), Hydrogen (H) and Nitrogen (N). The purpose of the present study is to develop a Kramers-Kronig exploration to analyze the infrared (IR) reflectance spectra from FTIR spectroscopy results for nanocomposites prepared by emulsion polymerization. The samples were characterized by means of FTIR, XRD, CHN, and TEM; finally, the phase change of the incident wave, the refractive index (real and imaginary), and the complex dielectric function were calculated in the wavelength range of 3500 - 2800 cm−1; the composition dependence of the optical features is discussed.
2. Experimental Procedure
2.1. Materials
In this study, the following materials were used as received without further purification. Methyl methacrylate (100.12 g·mol−1), Oleic acid (282.46 g·mol−1), sodium dodecyl sulfate (SDS 288.372 g/mol), ammonium persulfate (APS 228.2 g/mol), and ammonia solution (25%), all from Merck, and alumina nanoparticles from Scharlo, were used.
2.2. Characterization
FTIR characterization of the nanocomposite materials was accomplished using a Shimadzu (8400S) Fourier Transform infrared spectrophotometer. FTIR spectra were measured in the range of 400 - 4000 cm−1 with KBr. TEM analysis was performed with a PHILIPS microscope (accelerating voltage of 80 kV). XRD experiments were carried out using an XRD PHILIPS model PW1800. Finally, CHN elemental analysis was conducted by means of a Costech ECS 4010.
2.3. Nanocomposite Synthesis
In a typical procedure, first, the alumina nanoparticles were vacuum dried at 100°C to eliminate water. Then, using a proper OA/Al2O3 ratio, the surface of the alumina nanoparticles was modified by exposure under vigorous stirring to an OA/toluene solution at 50°C for 12 h. After surface modification of the Al2O3 particles, particles were dried again at 80°C for 5 h. The surface modified alumina particles (SMA) were then dispersed in methyl methacrylate (MMA) using an ultrasonic apparatus. Emulsification of the monomer-SMA mixture was performed using an aqueous solution of sodium dodecyl sulfate. Finally, emulsion polymerization was carried out at 80°C by adding ammonium persulfate as an initiator under a nitrogen stream for 3 h. The process was done for PMMA (without alumina), PMMA/15Al2O3 (P15A), and PMMA/25Al2O3 (P25A).
3. Results and Discussion
3.1. Grafting density calculations
Diverse OA/Al2O3 ratios were utilized in order to allow a choice of the highest grafting yield as the main composition. Based on the results of the CHN analysis, the carbon content difference of alumina and oleic acid grafted alumina after surface modification can be attained. The existence of carbon on the alumina nanoparticles after modification is due to surface modification with oleic acid. Furthermore, grafting density (GD) can be calculated from the following equation19):
where ΔC is the carbon difference between alumina before and after modification, Nc and M are the number of carbon atoms and the molar mass of oleic acid, and S is the surface area of the nano alumina particles (m2/g). The grafting yield, which corresponds to the fraction of OA grafted onto alumina, can be calculated using the following equation:
where [Oleic acid] (μmol·m−2) is the initial concentration of OA. The outcomes of the grafting density and the grafting yield are shown in Table 1.
As can be seen, the maximum grafting yield for OA/Al2O3 =1.5 is 2.018094. Therefore, this ratio was used to prepare the nanocomposites. The variations of GD and GY with the OA/Al2O3 weight ratio are shown in Fig. 1. There is a growing trend in GD and GY with increasing of the OA/Al2O3 ratio up to 1.5; the maximum value of GY is reached, and then GY diminishes abruptly, while GD increases slightly with increasing of the OA/Al2O3 ratio to 2.
3.2. FTIR analysis
Infrared spectroscopy is used for exploration of the structural configuration of materials, especially those including organic compounds, because of its proper sensitivity to infrared energy.
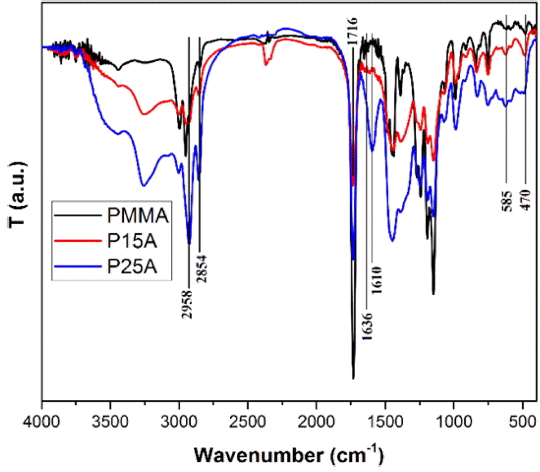
Figure 2 displays FT-IR spectra of PMMA and P15A, and P25A. The disappearance of the peak at 1636 cm−1 related to the C=C bond can be the result of good monomer conversion. There is a sharp peak at 1716 cm−1; this peak is associated with a carbonyl group (C=O) that is present in all samples because this bond does not change during polymerization. Stretch vibrations of the methyl groups are recognized as having a frequency of 2958 cm−1 ; this frequency is relatively stronger in the first sample (PMMA), while for the other samples it is broadened because of the formation of aluminum oleate. The existence of a bond between the aluminum ions and the carboxylate groups of oleic acid is confirmed by the 1610 cm−1 band, which cannot be seen in the pristine polymer. Since oleic acid contains a lot of CH2 groups, there must be an intensive peak associated with them in P15A and P25A. The peak at 2854 cm−1 in PMMA is weaker than those of P15A and P25A, which is the existence of oleic acid in the composite. Peaks below 1000 cm−1 correspond to Al-O stretching absorption; these include vibrations at 585 cm−1 and 470 cm−1, which are attributed to the vibrational stretching of the Al-O octahedral groups.20,21) In general, due to the incorporation of inorganic components, the absorption peaks of PMMA in organic groups are stronger than those in the nanocomposite.
3.3. XRD investigation
Powder X-ray diffraction (XRD) patterns of PMMA and PMMA/Al2O3 nanocomposites (P15A and P25A) are plotted in Fig. 3. Generally, the powder X-ray diffraction patterns in amorphous systems show broadened peaks, as can be seen for PMMA, which is entirely amorphous; consequently, according to JCPDS Card no. 13-0835, the diffraction pattern of PMMA shows the most intense peaks at 2theta values of 13°, 27°, and 41°. A comparison of the spectra of PMMA and PMMA/Al2O3 shows different behavior for the two materials.
It was observed that the intensity of the diffraction peaks in pristine PMMA meaningfully decreased as a result of alumina incorporation. In addition, the presence of new peaks was detected by the presence of alumina, as expected. The peak at 65° shows the presence of gamma alumina with cubic structure. An increasing crystallinity of PMMA/Al2O3 is observed with increase of the alumina content from 15 to 25% in terms of mass. The nano nature of samples was clear from the broadening peaks.
3.4. TEM investigation
Figure 4 shows the TEM images of PMMA, P15A, and P25A. Bonding between hydrophilic alumina and hydrophobic PMMA was created by means of the coupling agent oleic acid. The surface of the alumina nanoparticles was modified before polymerization; then, the surface modified particles were mixed with MMA and polymerization occurred.
It can be clearly observed that, because of the particles tendency to bond to each other, increasing the alumina content from 15 to 25%wt in the nanocomposite results in the appearance of agglomerates of alumina particles. The size of the fillers remained almost constant when the polymer shape was changed from spherical in the pure state to a shell-like round filler. Before polymerization, the surface of the particles was modified with oleic acid, which induces a C=C bond on the surface of alumina particles. During polymerization, this double bond can open and polymerization can take place on the surface of the fillers. Therefore, the above (Fig. 4) morphology for the nanocomposites can be achieved. It is possible to create a polymer shell around the filler particles, provided that sufficient coupling agents can bond to the surface of the inorganic constituents.
3.5. Optical constants determine by Kramers-Kronig analysis
According to the FTIR spectra and the following relations, some of the optical constants of the nanocomposites were calculated for a film of which the thickness is 70 μm. In order to use the Kramers-Kronig relation, the first reflection spectra of the samples must be withdrawn.
The phase change ϕ at a particular frequency between the incidence and the reflected wave is acquired from the KK dispersion relation:22)
The spectrum of ϕ (w) is used to obtain the real (n) and imaginary (k) parts of the complex refractive index, according to the following equations:23)
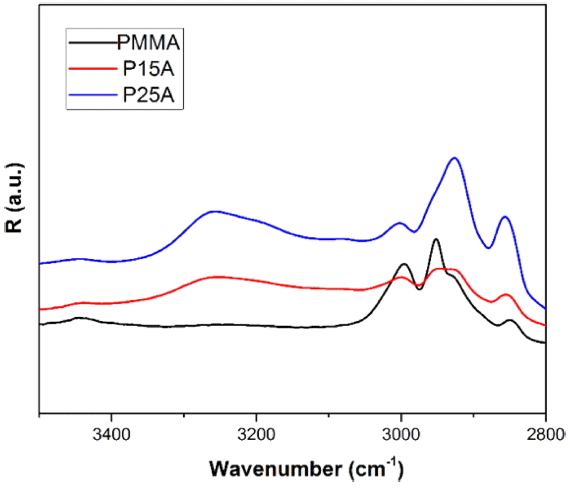
Based on Eqs. (3) and (4), simulated IR reflectivity spectra for nanocomposites were derived. The reflection of the samples in the range of 3500 - 2800 cm−1 is shown in Fig. 5.
As can be seen in Fig. 5, the reflection of IR in the range of 3500 - 2800 cm−1 has been increased; four peaks for reflection are obvious. The peaks at about 3250 cm−1 are assigned to hydroxyl groups on the surface of the aluminum oxide. Based on the reflection, and according to Eq. (3), the phase change of the incident wave across the nanocomposite versus the wavenumber in the range of 3500 - 2800 cm−1 is plotted in Fig. 6.
The total trend for samples is the same, except that in the vicinity of 2950 - 2850 cm−1 the impact of aluminum oxide on the behavior of the graph is remarkable.
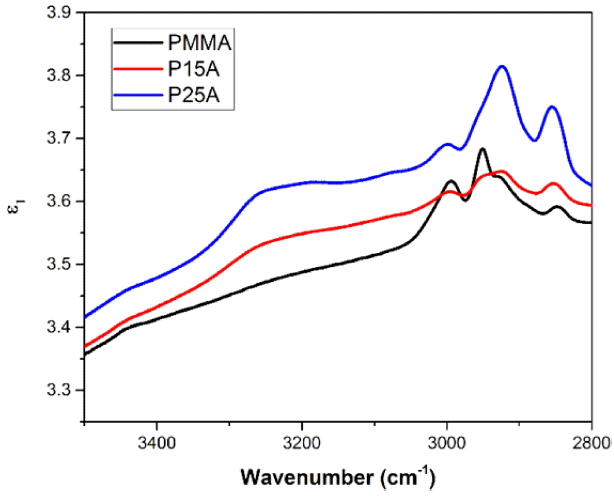
Based on the spectra of R(w) and ϕ(w), the spectra of n(w), k(w), ɛ1(w), and ɛ2(w) were obtained by numerical calculations.
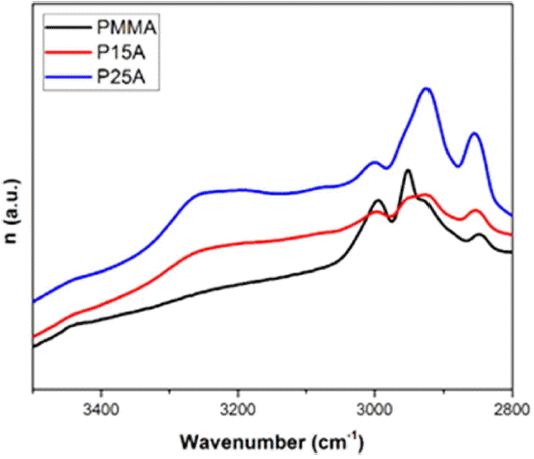
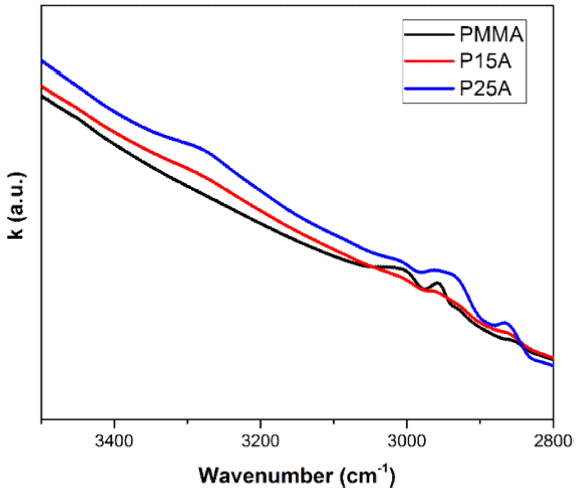
The real and imaginary parts of the refractive index are plotted in Fig. 7 and Fig. 8 in the range of 3500 - 2800 cm−1.
According to Fig. 8, the values of the imaginary part of the refractive index become higher with increasing alumina content for wavenumbers higher than 2850 cm−1; this may be due to excess hydroxyl groups in the alumina composites.
Subsequently, the real (ɛ1) and imaginary (ɛ2) parts of the complex dielectric function can be obtained by using:
4. Conclusions
In order to explore their optical constants, PMMA/Al2O3 nanocomposites were successfully prepared by emulsion polymerization with different amounts of alumina nanoparticles. The results of FTIR showed the presence of a chemical bond between PMMA and alumina. The existence of cubic gamma alumina was revealed by XRD. Increasing the filler content from 15% to 25% was found to result in agglomeration of the particles, which was confirmed by TEM images. We have offered a detailed explanation of the use of the Kramers-Kronig (KK) technique to examine normal incidence infrared (IR) reflectance spectra. In addition, KK was used to extract the optical constants, such as the complex refractive index and complex dielectric constants, by mathematical calculation.