1. Introduction
Piezoelectric ceramics, which have high ferroelectric and piezoelectric properties, are widely used in modern electronic devices such as sensors, actuators and memory devices.1,2) Lead-based ceramics such as PbZrO3-PbTiO3 (PZT) and PZT-based ternary compositions have excellent piezoelectric properties and are thus widely used in various piezoelectric devices. However, PZT-based piezoelectric ceramics contain more than 60 wt% of PbO, which is harmful to the environment. As a result, much research has focused on replacing the PZT-based piezoelectric ceramics with their lead-free counterparts.2-5) (Na0.5K0.5)NbO3 (NKN)-based piezoelectric ceramics have been extensively investigated in this regard, particularly since they exhibit promising piezoelectric properties and high Curie temperature (410°C).6,7) NKN ceramics have multiple, temperature-dependent structures. These include the tetragonal, orthorhombic, and rhombohedral structures that are stable below the Curie temperature, at approximately 200°C and below − 123°C, respectively.8-10) Many researches have been conducted with the goal of creating the rhombohedral and tetragonal phase boundary near room temperature in order to increase the d33 value of the NKN-based ceramics.10-12)
The 0.95NKN-0.05CaTiO3 (NKN-CT) piezoelectric ceramics have also been extensively investigated because they exhibit the promising piezoelectric properties.13) These materials also display the relaxor behavior, which decreases the temperature dependence of the piezoelectric properties.13) Adding CuO to NKN-CT ceramics results in a decrease in the sintering temperature to approximately 960°C and improved piezoelectric properties (d33 = 200 pC/N, kp = 0.37, ɛT33/ɛo= 1173, and Qm= 350).14) The d33 value of the NKNCT-based ceramics was lower than the PZT-based ceramics, indicating that it is difficult to use those materials in actuator devices. The g33 value of the CuO-added NKN-CT ceramics is approximately 20 × 10−3 Vm/N,14) which is close to that of the PZT ceramics used for the knocking sensor.15) NKN-CT ceramics can therefore be used in knocking sensors. However, NKN-CT piezoelectric ceramics have low resistance that stems probably from the evaporation of alkali ions. This evaporation gives rise to metal vacancies and holes, which makes poling of the NKN-CT difficult. Therefore, in this work, MnO2 was added to the NKN-CT ceramics in order to increase the resistance of the specimens and improve the poling efficiency of the material. The effect of MnO2 addition on the microstructure and the piezoelectric electric properties was also investigated in this work.
2. Experimental Procedure
0.95NKN-0.05CT ceramics were prepared from carbonates and oxides of > 99% purity using conventional solid-state synthesis. Carbonate and oxide compounds of K2CO3, Na2CO3, Nb2O5, CaCO3, and TiO2 (all from High purity Chemicals, >99.9%, Japan) were mixed for 24 h in a plastic jar with zirconia balls and then dried. The dried powders were calcined at 950°C for 3 h. MnO2 (High purity Chemicals, > 99%, Japan) was added to the calcined 0.95NKN-0.05CT, and then, the mixture was remilled and dried. The resulting powder mixture was pressed into discs under a pressure of 100 kgf/cm2 and sintered at temperatures in the range of 1000°C and 1060°C for various periods of time. In addition, structural properties of the specimens were examined by X-ray diffraction (XRD: Rigaku D/max-RC) and scanning electron microscopy (SEM: Hitachi S-4300, Japan). The densities of the sintered specimens were measured by Archimedes’ principle. A silver-palladium electrode was printed on the lapped surfaces of the polished specimens that were then poled in 120°C silicon oil at 4 - 5 kV/mm for 60 min. The polarization versus electric field (P-E) behavior was then determined by a modified Sawyer-Tower circuit. The piezoelectric properties and electromechanical coupling factor were measured by a d33 meter (Micro-Epsilon Channel Product DT-3300, Raleigh, NC, USA) and an impedance analyzer (Agilent Technologies HP 4294A, Santa Clara, CA, USA), all according to IEEE standards.
3. Results and Discussion
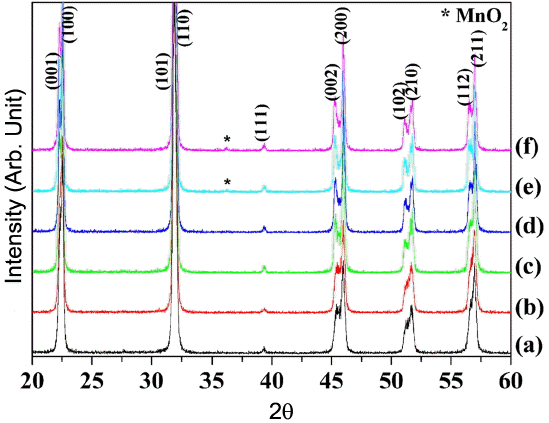
Figure 1 shows the XRD patterns of the 0.95NKN-0.05CT + x mol % MnO2 (0.0 ≤ x ≤ 2.5) ceramics sintered at 1020°C for 4.0 h. The homogeneous orthorhombic phase formed in specimens which have less than 2.0 mol% MnO2 and peaks of a secondary phase were not observed. However, peaks corresponding to the MnO2 phase (indicated by asterisk) were observed for the specimen with x≥2.0. The location of Mn ions is important since they influence the electric and piezoelectric properties of these materials. Mn ions exist as Mn4+, Mn3+, and Mn2+ at temperatures below 1000°C, above 1020°C and higher than 1200°C, respectively. Therefore, most of the Mn ions exist as Mn3+ in the 0.95NKN-0.05CT ceramics since sintering was carried out at 1020°C. With an approximate size of 0.7 A, Mn3+ ions may enter the B site of the 0.94NKN-0.06Ba(Zr0.05Ti0.95) ceramics, Mn3+ions can, however, entered both A and B sites and simultaneously play the role of donor and hardener.16) It is assumed, therefore, that the Mn3+ ions in the 0.95NKN-0.05CT ceramics may also enter the both A and B sites.
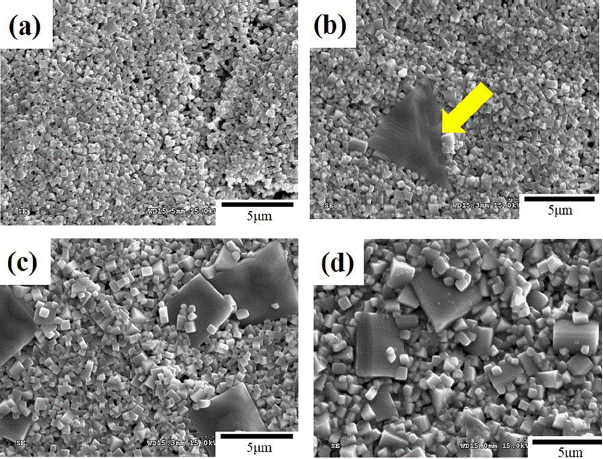
Figure 2(a) - (d) show the SEM images of the 0.95NKN-0.05CT + x mol % MnO2 (0.0 ≤ x ≤ 2.5) ceramics sintered at 1020°C for 4.0 h. The 0.95NKN-0.05CT ceramic is porous and fine grained, indicating that the 0.95NKN-0.05CT ceramic was not sintered at this condition. Adding 0.5 mol% MnO2 resulted in a similar structure to the case of x = 0, the major exception being the appearance of an abnormally large grain, indicated by the arrow in Fig. 2(b). Specimens with x ≥ 1.0 (Fig. 2(c)-(d)) developed dense microstructures with increased numbers of large grains. It is apparent from Fig. 2(c) - (d) that MnO2 additions lead to microstructural variations in the 0.95NKN-0.05CT ceramics. Furthermore, it was reported that alkali ions evaporate from NKN-based ceramics during sintering at temperatures higher than 1000°C.17,18) This evaporation results in the formation of an alkali ion-deficient liquid phase.19) Based on that reported observation, it was concluded that a liquid phase formed in the MnO2-added 0.95NKN-0.05CT ceramics sintered at 1020°C. The liquid phase was, however, insufficient in specimens with x ≤ 0.5, giving rise, therefore, to a porous microstructure. In contrast, MnO2 amounts exceeding 0.5 mol% result in dense microstructures with increased number of the large grains, owing probably to sufficient amount of the liquid phase. The amount of liquid phase increases with increasing MnO2 content. MnO2, therefore, promotes the formation of the liquid phase and contributes to the densification of the specimens.
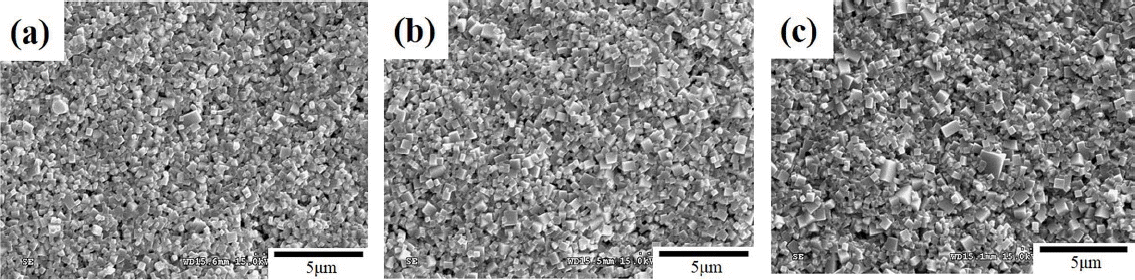
0.95NKN-0.05CT + x mol % MnO2 (0.0 ≤ x ≤ 2.5) ceramics sintered at 1060°C for 4.0 h were also examined by SEM (Fig. 3(a)-(c)). The microstructure consists of small grains whose formation is related to the amount of liquid phase present in the material. Sintering at 1060°C produced a large amount of the liquid phase, which is apparently important for the nucleation of abnormal grains. Nucleation of abnormal grains increases with increasing liquid content. These abnormal grains impinge on each other during the early stages of growth and inhibit the grain growth, leading to the development of a microstructure with small grains.17,20) The formation of the fine-grained microstructure in the MnO2-added 0.95NKN-0.05CT ceramics sintered at 1060°C can therefore be attributed to the presence of a large amount of the liquid phase.
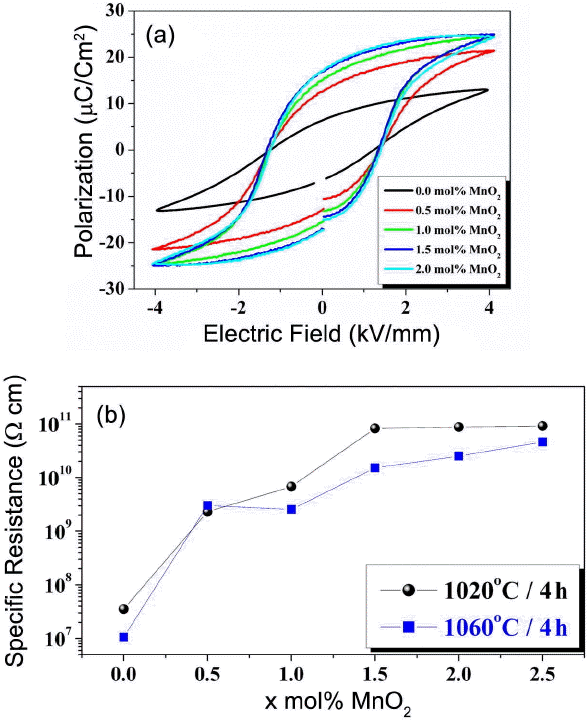
Figure 4(a) plots the P-E curves of the 0.95NKN-0.05CT + x mol % MnO2 (0.0 ≤ x ≤ 2.0) ceramics sintered at 1020°C for 4.0 h. The Pr value of the 0.95NKN-0.05CT ceramic was low, only approximately 5.9 μC/cm2, owing probably to the low density and porous microstructure of the specimen. Pr increases with increasing MnO2 content and saturates at 16.0 μC/cm2 for specimens with x ≥ 1.5 mol%. Ec, on the other hand, varies negligibly with increasing MnO2 content. This observation indicates that Mn ions result in negligible hardening effect in 0.95NKN-0.05CT. Furthermore, negligible hardening effect suggested that most of the Mn3+ ions entered the A-site and behaved as the softener, resulting in an increase of the Pr value.16)
Mn3+ ions located at A-sites also influence the resistance of the material. Fig. 4(b) shows the variation of resistance with MnO2 content of the 0.95NKN-0.05CT ceramics sintered at 1020°C and 1060°C for 4 h. The resistance of the 0.95NKN-0.05CT ceramic sintered at 1020°C was low, only approximately 3.5 × 107 Ω·cm. The resistance of the specimen increases with increasing MnO2 and saturates at a value of 8.27 × 1010 Ω·cm for x = 1.5. It was difficult to pole the 0.95NKN-0.05CT ceramic because of the leakage current stemming from the low resistance of the material. MnO2-added 0.95NKN-0.05CT ceramics were easily poled, however, owing to the increased resistance of these specimens. As stated previously, the evaporation of alkali ions during sintering results in the formation of metal vacancies in the 0.95NKN-0.05CT ceramics. These vacancies create holes in the ceramics, which decrease the resistance of the specimen, resulting in difficulties in the poling process. However, electrons are produced by Mn ions that act as donors when they enter the A-sites of the matrix. These electrons interact with the previously created holes, thereby increasing the resistance and improving the poling efficiency of the specimens (Fig. 4(b)). Specimens sintered at 1060°C also exhibited the increased resistance with increasing amount of MnO2. However, the resistance was lower than that of the specimens sintered at 1020°C, owing probably to the presence of a large amount of the liquid phase.
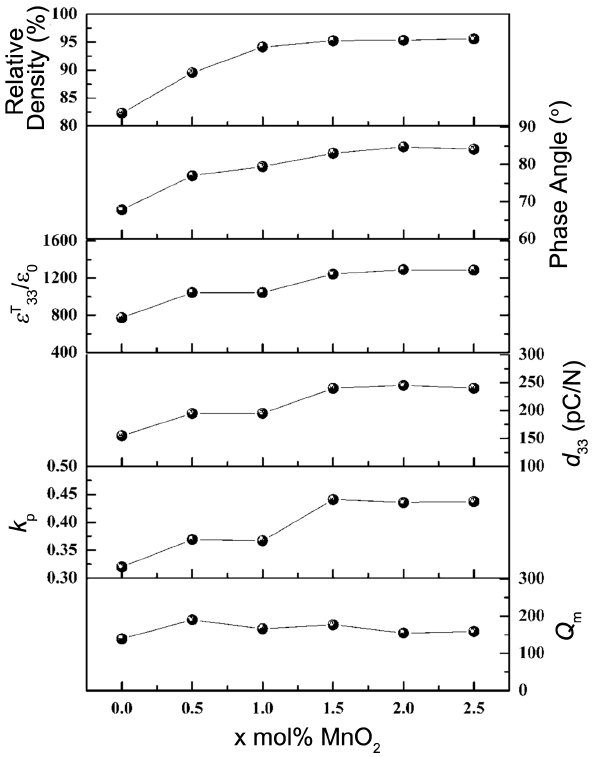
Figure 5 shows the relative density, phase angle, ɛT33/ɛo, d33, kp, and Qm values of the 0.95NKN-0.05CT + x mol % MnO2 (0.0 ≤ x ≤ 2.5) ceramics sintered at 1020°C for 4.0 h. The relative density of the 0.95NKN-0.05CT ceramic was very low approximately 82% of the theoretical density owing to the formation of the porous microstructure. Relative densities increase with increasing MnO2 and saturate at a value of 95% of the theoretical density for x = 1.5. This indicates that some of the MnO2 acts as a sintering aid and promotes the densification of the specimens. Phase angle of the specimens increased with increasing MnO2 content, indicating that the poling efficiency of the specimen improved with the addition of MnO2 probably owing to the increase of the relative density. The ɛT33/ɛo value of the 0.95NKN-0.05CT specimen was low (approximately 800) and, like the relative density, increased with the addition of MnO2. ɛT33/ɛo value saturates at some value (in this case 1200) when x=1.5. The increase of ɛT33/ɛo value with the addition of MnO2 is explained by the softening effect of Mn ions in the 0.95NKN-0.05CT matrix. Moreover, since the variation of ɛT33/ɛo is similar to that of the relative density, the ɛT33/ɛo could also be influenced by the density. d33, and kp also increase with increasing MnO2 content and Metal vacancies produced by the Mn3+ ion in A-site and the evaporation of alkaline ions might enhance the domain wall motion resulting in the improvements of the d33, and kp of the specimens. However, since the variations of d33 and kp values with respect to MnO2 content is very similar to that of the relative density, it is also possible that their enhancements could be influenced by the increase of the relative density. The 1.5 mol % MnO2-added 0.95NKN-0.05CT ceramic has saturated d33 and kp values of 240 pC/N and 0.44, respectively. The corresponding Qm value of the 0.95NKN-0.05CT ceramic is small owing to the low relative density and increases slightly to 180 when x=1.5. This value is slightly higher than that of the 0.95NKN-0.05CT ceramics sintered at high temperature.14) Based on this observation, it appears that a few Mn3+ ions also entered the B site and acted as the hardener. Therefore, the oxygen vacancies formed by the Mn3+ ion in B-site of the specimen hindered the domain movement resulting in the slight increase of the Qm value of the specimen.
4. Conclusions
The 0.95NKN-0.05CT ceramics were not sintered at 1020°C but adding small amount of MnO2 promoted sintering at this temperature. A dense microstructure consisting of small and also abnormally grown large grains were formed in MnO2-added specimens sintered at 1020°C. Although Ec remained almost constant, the Pr value of the 0.95NKN-0.05CT ceramics increased with the addition of MnO2. The resistance also increased resulting in improved poling efficiencies of these materials. It is assumed that most of the Mn3+ ions entered the A site of the 0.95NKN-0.05CT ceramics and acted as softeners, which increased the Pr value and resistance of the specimens. The relative density increased with increasing MnO2 content. Moreover, the ɛT33/ɛo, d33, and kp increased with the amount of MnO2 addition owing to the donor effect of the Mn3+ ions. The Qm also increased but only slightly, indicating that a very small amount of the Mn3+ ions entered the B site of the specimens and acted as the hardener. The 1.5 mol% MnO2-added 0.95NKN-0.05CT ceramic sintered at 1020°C for 4.0 h exhibited the most promising dielectric and piezoelectric properties with ɛT33/ɛo, d33, and kp values of 1216, 240 pC/N, and 0.44, respectively.