Flexible and Transparent Silica Aerogels: An Overview
Article information
Abstract
Silica aerogels are attracting attention due to certain outstanding properties such as low bulk density, low thermal conductivity, high surface area, high porosity, high transparency and flexibility. Due to these extraordinary properties of aerogels, they have become a promising candidate in thermal superinsulation. The silica-based aerogels are brittle in nature, which constrains their large scale-application. It is necessary to achieve transparency and flexibility of silica-based aerogels at the same time and with the same porous structure for optical field applications. Therefore, the present review focuses on the different sol-gel synthesis parameters and precursors in the synthesis of flexible as well as transparent silica aerogels. Also, a brief overview of reported flexible and transparent aerogels with some important properties and applications is provided.
1. Introduction
In recent years, the transparent and flexible materials have become required to produce low temperature thermal energy for various thermal insulation applications.1–5) Silica based aerogels are insulating materials exhibiting transparency and flexibility at the same time. The reason for the transparent nature of silica aerogels is the presence of silica particles smaller than the wavelength of visible light.6) Pores within this range can act as scattering centers, as explained by Rayleigh scattering. Fiber-like skeletons with smaller pores are essential for transparency via Rayleigh scattering and flexibility in aerogels that obtained from structural rearrangements and Ostwald ripening at high pH value.7,8) Also, the proper control of sol-gel parameters is important for improving the mechanical flexibility, hydrophobicity, and transparency of aerogels.9–12)
Silica aerogels and silica based materials prepared via solgel process have a wide range of applications.13–16) Especially, sol-gel glass can be used in variety of applications due to its high transparency.17) The term “sol-gel” was invented by Graham in 1864 during his experiment on silica sols.18) Silica aerogels prepared using sol-gel chemistry have been attracting considerable attention from scientists and engineers in different fields since their invention in 1931 by S.S. Kistler; these materials are fabricated by extracting pore liquid from wet gel using a supercritical drying approach.19) Silica aerogels are nanoporous materials with many unique properties such as low thermal conductivity, high porosity, hydrophobicity, high visible transparency, high specific surface area, and low dielectric constant.20–25) Due to the high level of porosity, with approximate pore size of 50 nm, aerogels are promising candidates for a large variety of applications in such areas as thermal insulation, catalysis, oil-spill cleanup, chemical sensors, interlayer dielectrics, drug delivery systems, and construction, as well as building insulation.26–32) The aerogels are applicable in thermal insulating windows due to their combination of visible transparency and low thermal conductivity; however, the fragile nature of silica is still an unavoidable barrier in attempts to achieve high mechanical strength of aerogels.
The most promising way to overcome this drawback is the formation of an organic-inorganic hybrid aerogel. In such a system, Si-O-Si contributes to the inorganic part and the Si-C bond represents the organic part; this combination brings about an improved hydrophobicity for aerogels and increases their mechanical strength.33) Hence, organic-inorganic hybrid aerogels are useful in special applications such as insulation at −40°C for space exploration.6) Nowadays, elastic and flexible aerogels are prepared using different strategies such as crosslinking polymers with organosilane compounds, surface modification, and using bridging alkoxysilane precursors.34–36) Also, many researchers have reported that the reinforcing of inorganic fibers, such as ceramic fibers, glass fibers, and mineral fibers, can improve the mechanical strength but not the flexibility, owing to materials essential brittleness.37–39) However, increasing the organic content causes structural inhomogeneity at nanoscale, which results in high thermal conductivity and opacity.40) Hence, a new category of thermal insulating aerogels, such as chitosan aerogels and cellulose aerogels, which have high flexibility and transparency, has been reported.41,42)
In the last ten years, a large number of articles has been published on the subject of aerogels, but very few reports are available on flexible and transparent silica based aerogels.43,44) This review paper aims to provide a comprehensive and timely overview of flexible and transparent aerogels, including their applications in different fields. Also, a systematic review of the effects of different parameters on the transparency and flexibility of silica based aerogels is provided.
2. Sol-gel Synthesis
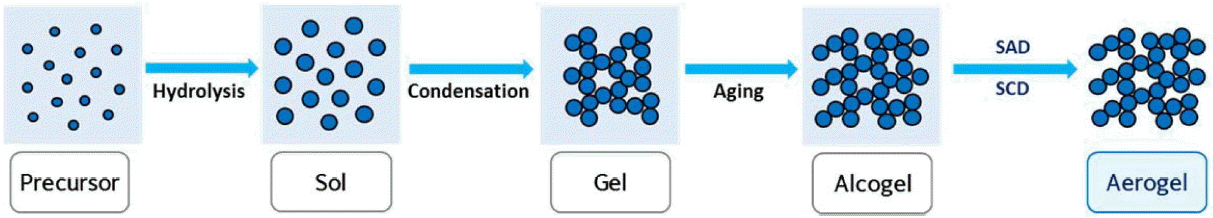
The detailed mechanism for the synthesis of silica aerogels can be found in books and in many reviews on aerogels.45–49) The sol-gel process is the main part of the synthesis of aerogels, though the precursors are inorganic salts or metal alkoxides. Mostly, the preparation of aerogels takes place in three steps, as follows:
Gelation: Sol to gel transition takes place via hydrolysis and condensation (sol-gel process)
Aging: Increase of the mechanical strength of the network formed during the sol-gel process
Drying: Removal of solvent at certain temperature and pressure with intact network
The schematic of the sol-gel process for the preparation of transparent and flexible silica aerogels is presented in Scheme 1. The transparency and flexibility of aerogels are affected by several parameters used in the sol-gel process, which are explained in the present section.
2.1. Some important parameters to control the transparency and flexibility
2.1.1. pH of solution
The hydrolysis of alkoxysilane starts with the addition of a catalyst, like an acid or a base, which is important for the sol-gel process. Therefore, the nature of hydrolyzed silica depends on the pH of the solution. For a low pH (strongly acidic) solution, the silica skeletons form a linear chain with very low skeletal density, which leads to the formation of a soft alcogel, which is then reversibly dispersed into the primary solution.50) The increase in the crosslinking of the network of the silica skeleton is possible at high pH (highly basic) solutions. At high pH, the silica network becomes strong due to greater branching of silicic acid, which forms due to the hydrolysis of alkoxysilane.50) In the case of low pH values, hydrolysis is due to electrophilic attack on the oxygen atom of the alkoxide group, while hydrolysis and condensation take place by nucleophilic attack on the Si atom at high pH values.51) At high pH, the stability of the PVSQ network against hydrolysis of the siloxane bond is not sufficient due to the strong electrophilic nature of vinyl groups, which nature affects the homogeneity during the aging step.52) Recently, some reports have become available on the advantages of strong acids and bases in processes to obtain transparent aerogels; these acids and bases promote additional condensation and rearrangement of the silica skeletons and help with the achievement of the essential microstructure. Structural rearrangement and Ostwald ripening occur easily at high pH values, which leads to fiber-like skeletons with smooth surfaces. It has been reported that the addition of urea accelerates the polymerization of methyltrimethoxysilane (MTMS), ethyltrimethoxysilane (ETMS), vinyltrimethoxysilane (VTMS), ethylene-bridged polymethylsilsesquixane (EBPMS), and bis(trimethoxysilyl) hexane BTMSH precursors by increasing the pH of the solution, which leads to a finer pore structure and results in transparent aerogels.52–55)
2.1.2. Aging
The most important parameter in the preparation of an aerogel is the aging of the alcogel, this process mechanically reinforces the tenuous silica network generated during the sol-gel process. Without aging, the transition from alcogel to aerogel is a very tedious job. Aging at certain temperatures strengthens the gel skeletons and minimizes the drying-stress induced volume shrinkage of the prepared aerogels.56) It was found that gelation proceeds by dissolving and depositing smaller segments on larger chains, which is nothing but Ostwald ripening. Aging takes advantage of syneresis and Ostwald ripening by altering the composition of the liquid phase present in the pores of the silica gel skeleton. In general, the apparent density and the average pore size of an alcogel can be changed according to the aging period and the aging temperature.57,58) The proper control of aging improved the mechanical properties of the prepared aerogels.59–62) Reports are available on the thermal aging of a wet gel in water; this became a key method to decrease the microporosity of the gel.63,64) Recently, successful aging treatments via the addition of polyethoxydisiloxanes and dilute HF solution have been reported to instantaneously increase the permeability and mechanical properties.65,66) The transparency of an aerogel depends on the concentration of the base catalyst and the aging temperature. In the case of strong basic condition, the condensation as well as the reverse condensation reaction of the siloxane bond is possible. Therefore, it is necessary to optimize the catalyst concentration and the aging temperature to obtain transparent and low density aerogels. In the case of the polyethylsilsesquixane (PESQ) and polyvinylsilsesquixane (PVSQ) systems, 60°C and 40°C aging temperatures are reported to make it possible to obtain transparent and flexible aerogels, 52) while the correct temperature for EBPMS aerogels is 80°C when a moderate concentration of base catalyst is used.54) Nadargi et al. reported on the effects of aging period on the flexibility of methyltriethoxysilane (MTES) based silica aerogels.67) The increase in the aging period of alcogels caused the separation of the silica chains, with large empty pores in the network structure; because of this, an increase in the porosity is observed.
2.1.3. Surfactant
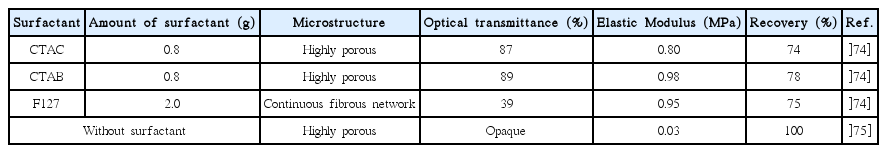
Sufficient understanding of the chemical reactions and of the interaction with other components during pore formation in the sol-gel process is required for rational and controlled design of porous materials. Periodic mesoporous organosilicas (PMOs) are prepared under controlled hydrolysis and condensation of alkoxides in the presence of surfactant.68–70) Surfactants are structure directing agents that advance the porosity of aerogels. Monolithic gels with micrometer size particle aggregates can be obtained in the absence of any surfactant as a result of the phase separation of methylsiloxane (MSQ) networks from silica sol.71) On the other hand, Kanamori et al. for the first time synthesized transparent aerogels from MTMS by using a surfactant to control the macroscopic phase separation.72) For this, two kinds of surfactants, including cationic and non-ionic tri-block copolymers, were found to successfully suppress the phase separation.73)
The amount of surfactant plays an important role in the formation of transparent as well as flexible aerogels. Table 1 provides a brief overview of the MTMS based aerogels prepared by employing different types of surfactant and without the use of surfactant. In the absence of surfactant, only an opaque macroporous gel skeleton is obtained due to the phase separation of the hydrophobic condensates. If the amounts of surfactant are increased from 0 to 0.8 g (CTAB and CTAC) and from 0 to 2 g (F127), the transmittance increases and pore structures become finer, with nanometer pore size, which is less than the wavelength range of visible light. For high concentrations of surfactant, the reduction in the phase separation causes a decrease of the domain size, as well as an increase in the light transmittance; in this region, the shrinkage is higher, and this causes inhomogeneity and decreases in the transparency. This is due to the presence of a loose and softer network between the pore skeletons due to the increase in the surfactant. Further increases in the surfactant amount can offer more complexity, with MSQ condensates with looser networks.
The transparent PMSQ systems are reported to employ various types of surfactants, such as Pluronic F127, P105 and cationic n-hexadecyltrimethylammonium bromide (CTAB), and n-hexadecyltrimethylammonium chloride (CTAC). In the cases of the PESQ and PVSQ systems, EH-208 surfactant was used because it acts as a solvent, increasing the compatibility of the silica condensates.52) For bridged silica precursors, a volume fraction of EH-208 higher than 0.12 was found to result in high transparency due to the suppression of macroscopic phase separation. To avoid any decrease in the transparency, and to avoid volume shrinkage, it is also important to optimize the concentration of the precursor and the aging temperature.
2.1.4. Drying
The process of removing a solvent from an alcogel without disturbing the network is nothing but drying. Aerogels are typically prepared by extracting the solvent in supercritical state; this can be accomplished by achieving the supercritical state of the solvent used. Supercritical drying can be further divided into two types according to the critical temperature of the solvent used, as follows: (high temperature and pressure) supercritical alcohol drying (SAD)76) and (low temperature and high pressure) supercritical CO2 drying (SCD).77) At critical temperature and pressure, the liquid used reaches a supercritical fluid state, in which the surface tension of the liquid becomes zero to obtain monolithic aerogels.78)
In the SAD process, alcohols like methanol, ethanol, and propanol are commonly used because the critical temperatures of these fluids are greater than 220°C. The schematic setup for SAD is presented in an earlier review.46) Several reports are available on flexible as well as transparent silica aerogels prepared using the SAD method.75,79–81) Rao et al. reported flexible but opaque silica aerogels obtained using MTES and MTMS precursors followed by methanol drying.67,75) To reduce the time required to prepare aerogels by SAD, our group has reported a rapid supercritical extraction process that employs a hot processing method in which gelation, aging, and drying take place in a single step.76) Although this method is useful to prepare monolithic aerogels, there are some limitations due to the high temperature and pressure. Hence, nowadays, an alternative SCD technique is used for the preparation of transparent and flexible silica aerogels; this technique is inexpensive and has low temperature and pressure relative to SAD.52,54)
SCD is a commonly used low-temperature drying technique with a critical temperature of only 31°C. Compared to SAD, this method is safe as well as energy-efficient, because it requires non-explosive CO2 gas. In earlier times, there was a problem of shrinkage during SAD due to a restructuring of the clusters.82) This problem was later solved by Loy and co-workers; they synthesized silica aerogels directly in supercritical CO2.83) Also, a typical approach for drying an alcogel in continuous flow of supercritical CO2 (SC) was reported.84) In this case, the wet gel is loaded into an autoclave and dried in a continuous flow of SC. Kanamori et al. reported PMSQ, ethylene, and hexylene bridged transparent as well as flexible silica aerogels prepared using a two-step sol-gel process followed by SCD.52,54,55) In this synthesis, the pore liquid was completely replaced by SC and the alcogel was kept inside the autoclave for 10 h at the 80°C critical temperature and 14 MPa critical pressure.
3. Aerogels Using Organosilane Precursors
3.1. Flexible aerogels
At present, RSi(OR’)3 and Si(OR’)4 precursors are largely used in the preparation of transparent and flexible silica based aerogels; the notation R is an alkyl group. These starting materials are characterized by the existence of polar covalent bonds Si-O-Si in their molecules. Compared to other metal alkoxides, silicon based precursors are much more extensively used due to their excellent physical and chemical properties, as well as their applications in several fields. Silica aerogels prepared by tetraethoxysilane (TEOS), tetramethoxysilane (TMOS) are brittle and highly hydrophilic, which limits their applications.85,86) Hence, the use of an inorganic-organic hybrid system is one way to avoid problems: in such a system, Si-O-Si linking contributes the inorganic part and the Si-C bond contributes the organic part, leading to an increase in the hydrophobicity as well as the mechanical strength.
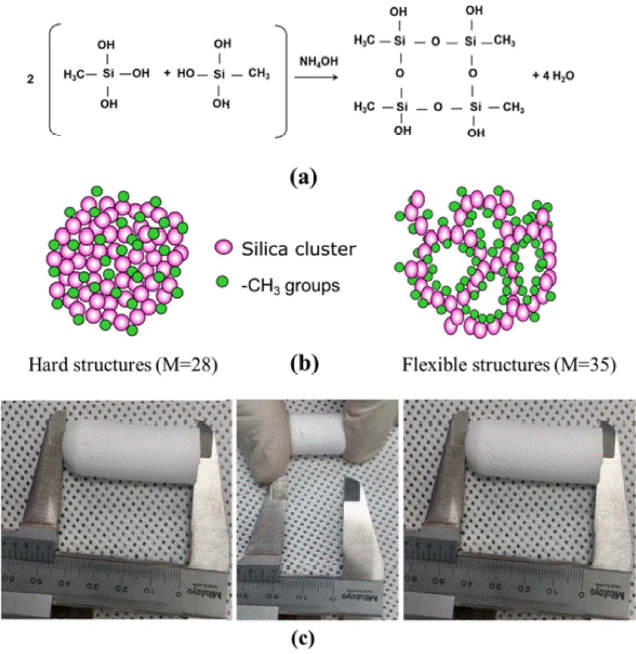
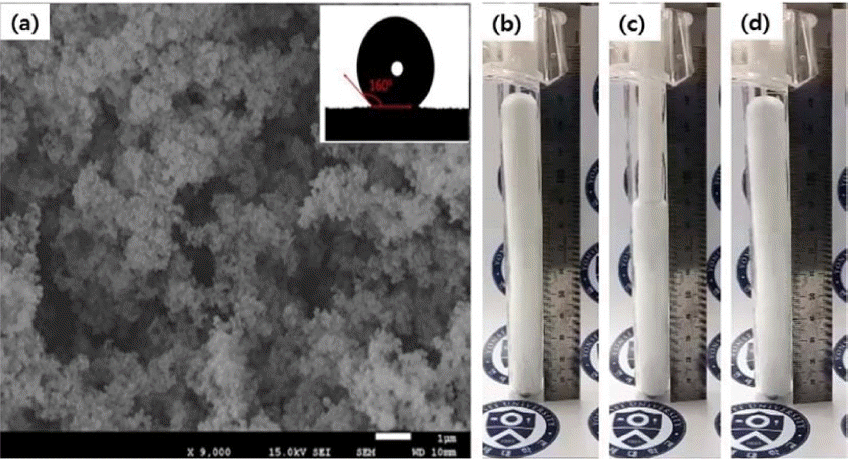
Nowadays, in the aerogel field, flexible silica aerogels using tri-functional organosilane precursors are emerging. MTMS based flexible and superhydrophobic aerogels have been reported by our group.87) Fig. 1(a) shows the condensation reaction of MTMS monomers; a schematic of the hard and flexible aerogels prepared with M = 28 and M = 35 is given in Fig. 1(b). Actual images of elastic aerogels are provided in Fig. 1(c). To increase the flexibility of the silica aerogel, the particular solvent to precursor ratio should be maintained. In the MTMS precursor, each monomer has one non-hydrolysable methyl group and three non-hydrolyzable methoxy groups.88) Therefore, only the hydrolysable methoxy groups are responsible for the matrix formation, this process is followed by hydrolysis and condensation. These MTMS based aerogels show 60% compressibility and deformation behavior. This type of elastic and superhydophobic aerogels, with water contact angle of 160°, as observed in the inset of Fig. 2, can be applicable as shock absorbing materials. Rao et al. has reported preparation of highly flexible and superhydrophobic silica aerogels using MTMS by two-step acid-base catalyzed sol-gel process followed by SAD.75) The minimization of inter-chain cohesion between MSQ particles resulted in an elastic and flexible silica network.89) The silica clusters are distributed homogeneously and uniformly in the network, as shown in Fig. 2; also, due to MTMS, the silica clusters used in this study contain hydrolytically stable methyl groups, which increase the hydrophobicity of the aerogels (inset of Fig. 2). Although these aerogels are opaque due to macroscopic phase separation, their flexibility is noteworthy. The increase in pore size shown in Fig. 2 caused more flexibility, because the probability of cracking decreased due to the minimization of the volume density between the connecting points of the silica particles.89) It was observed that the prepared aerogel sample could be compressed to around 80% of its original volume and then regained its original dimensions after the removal of the applied stress. In the cases of these aerogels, Young’s modulus increased with the increase in the bulk density, which is an ideal relation; these aerogels were superhydrophobic, with water contact angle of 164° and with thermal stability at around 530 K. Many groups have observed the same tendency for the MTMS based aerogels, as reported in.90–92) The demonstration of flexible MTMS-based aerogels is shown in Fig. 2(b–d). Superhydrophobic and flexible aerogels using MTES as a single precursor were reported by Rao et al..67) The prepared aerogels were highly elastic and flexible, with Young’s modulus of 3.95 × 104 N/m2. Also, additional propyl groups in the underlying silica network of the MTMS based aerogels can improve the flexibility of silica aerogels with 70% compressibility.93)
Aravind et al. reported new flexible aerogels using MTMS and 3-(2,3-epoxypropoxy) propyltrimethoxysilane (GPTMS) as co-precursor.93) In several inorganic-organic hybrid materials, GPTMS was used as a common precursor and the hybrid materials prepared using this precursor found applications in antifogging and anticorrosion coatings.94–96) To avoid phase separation of the two different organofunctional alkoxides, (1-hexadecyl) trimethylammoniumchloride (CTAC) surfactant was employed. All samples were flexible, with Young’s modulus of 0.46 MPa. Similarly, using MTES and PDMS as co-precursor, the two-step acid-base catalyzed solgel process was adopted by Zhong et al. for the preparation of highly flexible aerogels.97) Disilanol-terminated PDMS was used to improve the flexibility of silica aerogels. Co-polycondensation of terminal silanol groups of PDMS and hydrolyzed MTES can take place under basic conditions to achieve a flexible network.
Li et al. prepared flexible composite silica aerogels reinforced with aramid fibers (AF) to meet specific requirements of certain industrial applications.98) AFs were used as the reinforcement phase because, compared with other inorganic fibers, this material has low density, low thermal conductivity, and higher mechanical strength.99,100) Also, the decomposition temperature of aramid fibers is higher than that of other organic fibers. Due to this, AF reinforced flexible aerogels are applicable for high temperature thermal insulation. The increase in the AF contents in silica aerogel induces increase in the bending strength and modulus. The 5% AF contents was an optimized value chosen to allow better flexibility and lower thermal conduction in the aerogels.
Several researchers have used MTES as a primary precursor to synthesize silica based aerogels, as well as to synthesize hydrophobic coatings.67,79,101) Yu et al. have reported MTES based flexible, hydrophobic, and oleophilic aerogels synthesized through a two-step acid-base catalyzed sol-gel process.102) The prepared aerogel has low density (0.046 g/cm3) and 80% compressive strength. These aerogels show maximum bending without cracks and recover their original size rapidly. The hydrophobicity of the reported aerogels remains unchanged after they are washed several times with water. Hence, these materials were found to float on the surface of water. As is well known from many reports, MTMS has been used as a single precursor in the one-step base catalyzed sol-gel process to obtain PMSQ aerogels and coatings with micrometer size pores; these aerogels have been found to be supehydrophobic in nature.78,91,103,104) Also, MTMS based flexible aerogels with density of approximately 0.037 g/cm3 have been prepared by two-step acid-base catalyzed sol-gel process in methanol followed by SAD.105) These aerogels were opaque in nature due to their macroporous network, which is in the micrometer range; this network resulted in macroscopic phase separation of MTMS and methanol. Hence, several results have been reported on attempts to control the phase separation tendency of MSQ condensates.7,52,54,72)
Over the past three years, through a facile one-pot reaction, researchers from Kyoto University in Japan have succeded in the preparation of marshmallow-like gels derived from MTMS and Dimethyldimetthoxysiane (DMDMS).90) The design of flexible gels with different functional groups, without following any complicated process, was attempted by this group. Compared with conventional organic polymers such as polyurethene and polyethylene, the MTMS-DMDMS gel showed higher flexibility over a wider temperature range due to their network-like PDMS.90,106) These gels can recover their original shape after an 80% uniaxial compression and bending test. The bending deformation of MTMS-DMDMS aerogels and their stress-strain behviour is shown in Fig. 3. Several reports are available on aerogels and coatings using tetra and tri-functional alkoxysilane precursors and also using tetra-functional alkoxysilane and phenylalkoxysilane107–110); however, very few researchers have reported on flexible aerogels using tri- and dialkoxysilane, due to the high tendency of phase separation that results from the high hydrophobicity of the network.90,106)
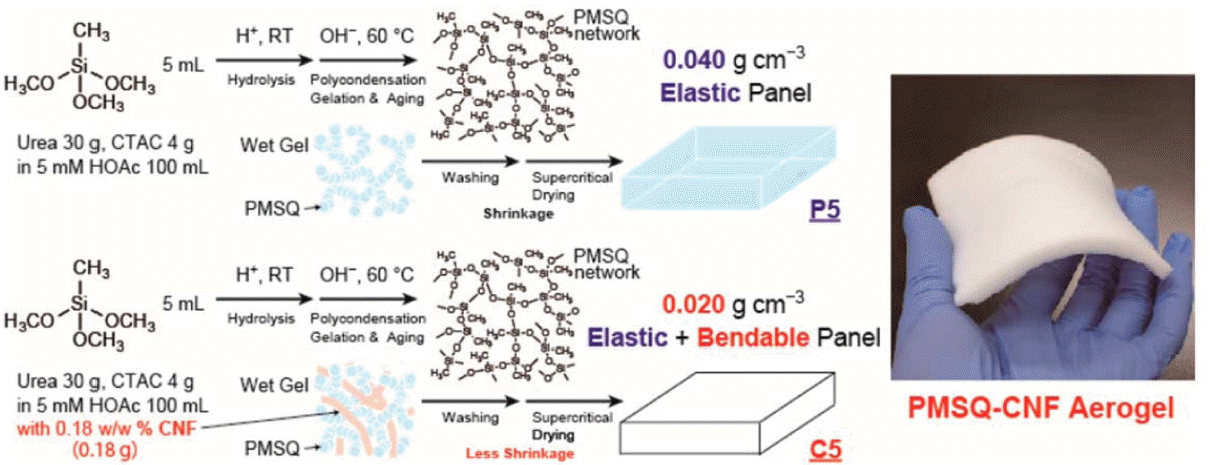
To improve the mechanical robustness, silica-bionanofiber composite aerogels have been investigated.111) For this, due to their abundance in nature and their excellent mechanical properties, cellulose nanofibers (CNFs) have been used.112,113) The mechanical strength of CNFs is approximately five times higher than that of steel wires.114) For this reason, these composite aerogels showed higher mechanical strength and flexibility against bending. CNFs synthesized from scrap wood can reduce the cost and environmental load in industrial applications. It was reported that the mechanical properties of PMSQ aerogels were improved by integrating CNFs. The synthesis process for CNFs/PMSQ composite aerogels and an image of the as-prepared flexible PMSQ-CNF aerogel is presented in Scheme 2.
Flexible aerogel composites, with a diameter of 12 cm, were successfully synthesized via electrospinning and solgel process.115) The composite aerogel, reinforced with electrospun PVDF nanofibers, had the lowest thermal conductivity and high mechanical strength. The prepared composite had a thermal conductivity of around 0.027 W/m·K. On the other hand, Cakmak et al. reported flexible aerogels using PDMS and electrospun PU/PEO nanofiber as reinforcement. For this synthesis, the authors have used a novel film casting/electrospinning process. The developed hybrid flexible aerogels have thermal conductivity of about 0.0129 W/m·K, which makes them useful in applications such as flexing, wrapping, and rolling. The main problem of all flexible aerogels is their opaque nature, which can be controlled using several sol-gel parameters, as has been discussed. In accordance with this, it is necessary to discuss the transparency and flexibility of aerogels. So, the next section will give a brief review of published results for flexible and transparent silica based aerogels.
3.2. Flexible and transparent aerogels
The only limitation in the use of aerogels has been their brittleness, which is caused by the high porosity and low connectivity of each colloidal particle. This creates difficulty in drying wet gels or in handling the prepared aerogels without damage. In order to obtain transparent and flexible aerogels, there should be a 30 – 40% limit for the incorporation of trifunctional species with respect to tetrafunctional monomer. Kanamori et al. prepared transparent and flexible aerogels and aerogel-like xerogels based on MSQ using one-step sol-gel process with MTMS as starting precursor.43) These aerogels demonstrated sponge-like flexibility during uniaxial compression.
The MSQ aerogels reported by Kanamori et al.43) showed a dramatic improvement of the mechanical durability against compression. It was observed that the gel shrank up to 80% in linear scale and sprang back to the original size when unloaded. In the case of pure silica aerogels like this, significant deformation has not observed; this is attributed to three different factors in the MSQ networks. The first fact is that having only three siloxane bonds per silicon atom lowers the crosslinking density and makes the macroscopic gel more flexible than pure silica aerogels. Second, the low density of the silanol groups hinders the nonreversible shrinkage. In pure silica aerogels, permanent shrinkage was observed to occur because of silanol groups with high density forming additional siloxane bonds due to drying stress; this was avoided by using MSQ. The third factor was that, in the high density network, homogeneously distributed methyl groups would repel each other during temporal shrinkage upon compression. These three factors contributed to the significant deformation of the prepared aerogel samples.
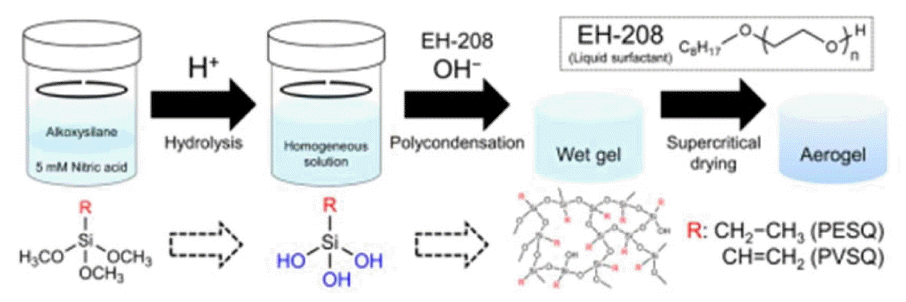
The synthesis of flexible and transparent aerogels using only organotrialkoxysilane was difficult because the hydrophobicity of the siloxane condensates increased with the polycondensation reaction.116) In this case, opaque PMSQ aerogels were obtained because phase separation between the solvent and the condensates took place before gelation. Hence, in the presence of surfactant, it is necessary to use a two-step acid-base catalyzed sol-gel process to obtain transparent and flexible aerogels. The addition of an acid catalyst caused the homogeneous hydrolysis to progress, and the addition of a base catalyst accelerated the polymerization of the silanol groups, which led to gel formation. On the other hand, the presence of a surfactant allows us to avoid the macroscopic phase separation between the alkoxysilane precursor and the polar solvent. Finally, the optimization of these different sol-gel parameters can be used to produce transparent and flexible silica based aerogels. Recently, reports are available on PESQ and PVSQ aerogels and xerogels synthesized using a two-step acid-base catalyzed sol-gel process followed by SCD and evaporation drying methods.52) These researchers have introduced a new two-step sol-gel process that was designed to form PESQ and PVSQ aerogels, which exhibit high visible-light transparency as well as mechanical strength.
The procedure for the synthesis of the PESQ and PVSQ aerogels is presented in Scheme 3. The different synthesis parameters affecting the transparency were as follows: water/precursor molar ratio, surfactant concentration, and base catalyst concentration. The reported transparent aerogels were obtained at higher concentration of base catalyst, which was the main variable that had to be regulated to achieve transparency in the silica based aerogels.
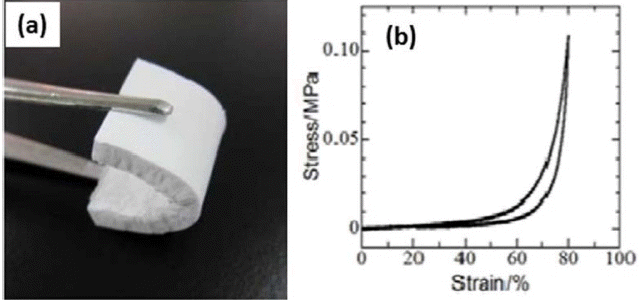
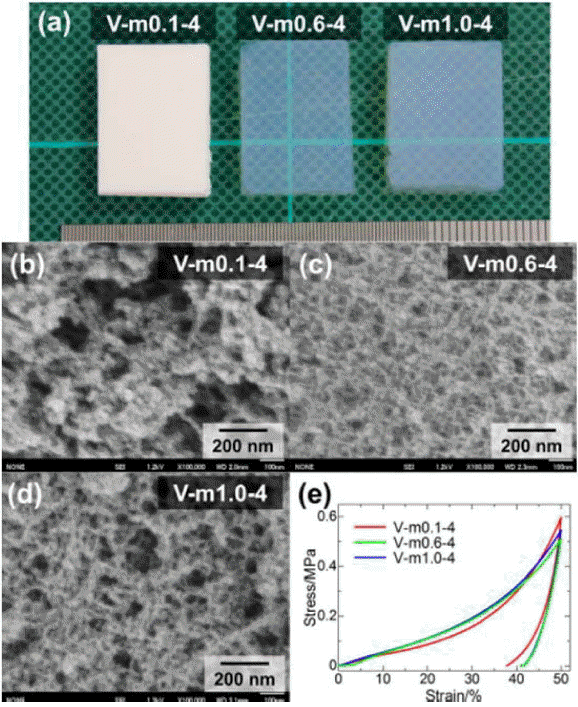
The morphological and mechanical properties of PESQ aerogels are shown in Fig. 4. Images of the as-prepared samples can be observed in Fig. 4(a) and are denoted by E-e0.1-40 and E-e1.5-60, where E is precursor, and e0.1 and e1.5 represent the concentration of tetraethylammonium hydroxide (TEAOH) with 40°C and 60°C aging temperatures, respectively. Differences in the microstructure were observed due to the different concentrations of the base catalyst used for the synthesis of the aerogels. In the case of sample E-e0.1-40, the coarsened structure resulted in an opaque nature due to strong visible light scattering according to Mie scattering phenomena.117) However, another sample showed smaller pore skeletons and an increased contribution of Rayleigh scattering, which resulted in high transparency.118) These differences in the morphology and optical nature were due to variation in the concentration of the base catalyst used. The mechanical properties of PESQ aerogels are determined using stress-strain curves, shown in Fig. 4(e); it can be seen that 50% compression is demonstrated by both samples without break. Fig. 5 provides images of the PVSQ aerogels with variation in the base concentration denoted by m. In the PVSQ aerogels, a similar effect of the base catalyst on the transparency is reported. The increase in the concentration of TMAOH brings a dramatic change in the appearance, from opaque (V-m0.1-4) to transparent (V-m1.0-4); again, transparency decreased for V-m1.0-4, as shown in Fig. 5. This change in transparency was caused by the coarsening due to the dissolution-reprecipitation mechanism at high basic conditions.119)
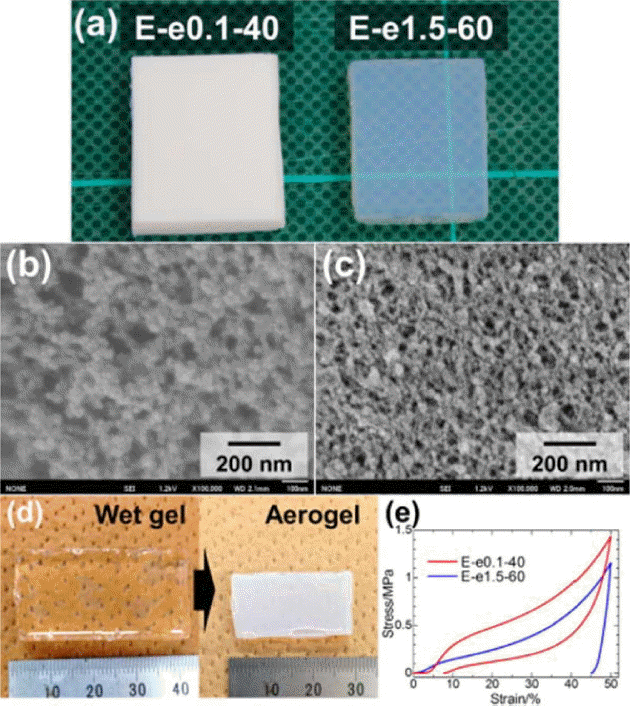
(a) Appearance of PESQ aerogels E-e0.1-40 (left) and E-e1.5-60 (right); FE-SEM images of PESQ aerogels (b) E-e0.1-40 and (c) E-e1.5-60; (d) Large volume shrinkage after supercritical drying of the sample E-e1.5-40; (e) Stress-strain curves on uniaxial compression-decompression of E-e0.1-40 and E-e1.5-60. Reprinted with permission from.52) Copyright (2016) American Chemical Society.
Organobridged alkoxysilanes are silane precursors with organic groups pre-installed through stable C-Si bonds, like (R’O)3Si-R-Si(OR)3 and (R’O)2CH3Si-R-SiCH3(OR’)2, where R represents an alkyl or aryl group attached with silica clusters to form a bridged network. Bridged polysilsesquioxanes networks composed of two silicons interconnected with a three siloxane bond can provide a more hydrophobic structure.120) These types of precursors were used to prepare PMOs and are useful to improve the mechanical properties of materials.121,122) Several researchers have reported the preparation of aerogels from bridged precursors. In the case of phenylene and hexylene bridging, PSQ aerogels with improved mechanical strength were prepared.123) Also, surface modification using hexamethyldisilazane (HMDS) was found to improve the hydrophobicity and mechanical strength of the prepared aerogels.124)
In some reports, flexible but opaque aerogels were prepared using 3-mercaptopropyltrimethoxysilane125) and VTMS/MTMS91); enhanced phase separation due to the presence of hydrophobic organic bridging groups was reported for these materials. Also, Nguyen et al.126) reported flexible aerogels fabricated using BTMSH and VTMS crosslinked with polysterene, while Guo et al.127) used bis[3-(triethoxysilyl)propyl] disulfide, tetramethylorthosilicate, and vinyltrimethoxysilane to achieve the same materials. Though these materials are opaque in nature, the combination of flexibility and hydrophobicity is useful in oil/water separation. Recently, using one pot co-condensation approach with the aid of a surfactant, Ehgartner et al. prepared flexible organofunctional aerogels with relatively large functional moieties.128) The samples showed excellent compression behavior with 60% compressibility.
Using the sol-gel process in N,N-dimethylformamide solvent, low density and transparent aerogels have been for the first time prepared using 1,6-bis(trimethoxysilyl)hexane followed by SCD.55) Compared with PMSQ aerogels, the resultant hexylene-bridged PSQ aerogel shows improvement in bending ability, with limited unloaded resilience, due to the high amount of silanol groups present on the surface after gelation. The resulting aerogels were post treated using HMDS to minimize silanol groups, which hinder the shrinkage-reexpansion behavior on compression-decompression. The transparent (56% at 550 nm) aerogels obtained using SCD, with high surface area (874 m2/g) and low density (0.18 g/cm3), as well as evaporative drying result in a monolithic xerogel with good transparency (71% at 550 nm) and low density (0.13 g/cm3).
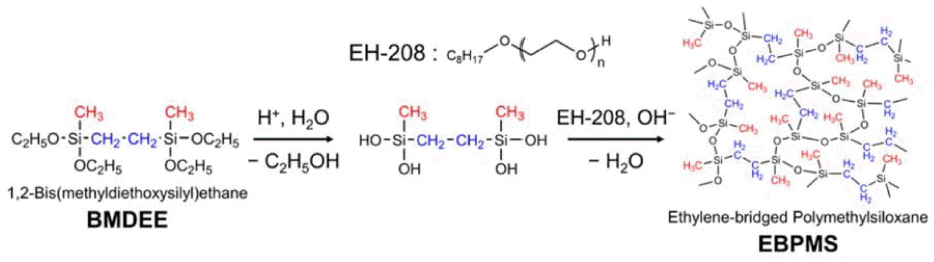
In (R’O)2CH3Si-R-SiCH3(OR’)2 type bridging precursors, each silicon atom has one organic bridging group and three siloxane bonds that correspond to substitutions of silicon atoms. The replacement of one siloxane bond with a methyl group causes improvements in the hydrophobicity and mechanical strength of the obtained aerogels. To the authors’ knowledge, only a few studies on transparent and flexible silica aerogels using bridged precursors have been reported so far.54,55) Loy et al. reported hydrophobic mesoporous gels with high specific surface area (~ 1000 m2/g) prepared using hexylene and phenylene bridged precursors.123) Specific control of the condensates and the silica network at nanometer scale, with better homogeneity to suppress Mie and Rayleigh scattering, is required to obtain transparent aerogels. Recently, Shimizu et al.54) reported flexible and transparent aerogels synthesized using a bridged polymethylsiloxane precursor such as 1,2-bis (methyldialkoxysilyl) ethane (BMDEE), which uses pendant methyl groups to achieve hydrophobicity. Surface modification was not required in this case, because the methyl groups present in BMDEE induced hydrophobicity in the prepared aerogel samples. A schematic for the preparation of an EBPMS network is shown in Scheme 4; this schematic is analogous to that of the PMSQ network except that one third of the siloxane oxygen is replaced by ethylene, which causes an improvement in the microscopic as well as the mechanical properties of the aerogels.
In this preparation, a two-step acid-base catalyzed sol-gel process was employed with surfactant to suppress the phase separation between BMDEE and polar solvents. The transparency and volume shrinkage varied with the base catalyst concentration. The proper replacements of siloxane bonds with organic bridges enhanced the flexibility against various deformation modes. Images of the as-prepared EBPMS aerogels, with a demonstration of the bending deformation, are given in Fig. 6.
Many researchers have reported the synthesis of mechanically flexible and strong silica aerogels by combining silica precursors with polymers,130–132) but the decrease in transparency with the addition of polymer has limited the applications of these aerogels. Recently, researchers at the University of Tokyo have reported a novel kind of polymer-silica hybrid aerogel fabricated via one-pot sol-gel process; for this preparation, the polymer used was polyrotaxane.129) As shown in Fig. 7(a), polyrotaxane is a mechanically interlocked linear polymer threaded into cyclic molecules. Since cyclic molecules cover the chain of the polymer, these aerogels are flexible as well as transparent (Fig. 7(b)) due to crystallization and reduced aggregation of polymer. The cyclic molecules in polyrotaxane can slide on the polymer chain; therefore, it is known as a “slide-ring material”, in which crosslink points can slide along the polymer chain by relaxing internal stress, in the way of a pulley system. Fig. 7(c) shows typical stress-strain curves of pure TEOS-based silica and polyrotaxane-silica hybrid aerogels; the differences between the two are immediately evident. These aerogels are elastic under small compression strains and can withstand more than 10 MPa of compressive strength with ~ 70% maximum strain without cracking of samples. The demonstration of the compressibility of the hybrid silica aerogels is presented in Fig. 7(d). Compared to other hybrid aerogels, these polyrotaxane-silica hybrid aerogels can be useful in thermal insulation applications due to their combined low thermal conductivity and high mechanical strength.
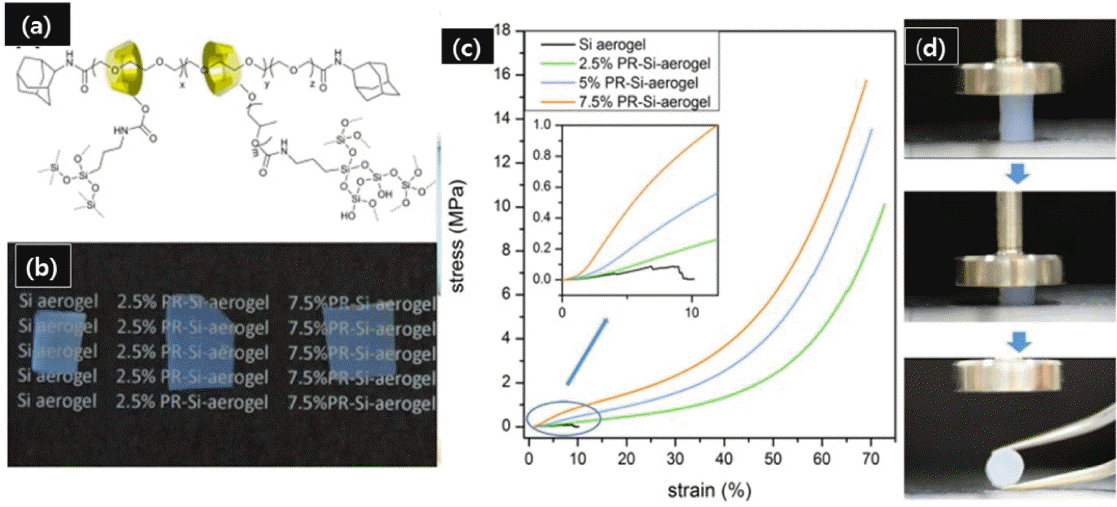
(a) Structure of silica nanoparticle grafted polyrotaxane, (b) Optical images of pure silica aerogel, 2.5% PR-silica aerogel, and 7.5% PR-silica aerogel, (c) Stress-strain curves of the Si-aerogel and polyrotaxane hybrid aerogels under compression tests, and (d) Compression process of 7.5% PR-Si aerogel. Reprinted with permission from.129) Copyright (2017) American Chemical Society.
4. Thermal Insulation Applications of Flexible Aerogels
Flexible aerogels are promising materials for a variety of thermal insulation related applications, such as building insulation, cryogenics, and vacuum insulation.133,134) The properties, such as high surface area, sharp pore size distribution, very low thermal conductivity, high flexibility, and transparency, can make these materials useful for different industrial and energy related applications. An overview of the important properties of some reported flexible silica aerogels is given in Table 2. The application of flexible and transparent aerogels in buildings as well as in cryogenic insulation is explained in the present section.
4.1. Building insulation
For building insulation, due to their low thermal conductivity and high temperature insulation performance, flexible and transparent silica aerogels are an alternative to traditional insulation materials. Reports are available on the application of transparent aerogels for daylighting purposes in new buildings.135)
Recently, Aspen Aerogels, Inc., developed an aerogel based insulation material that is known as Spaceloft®.136) This is a flexible aerogel blanket with room temperature thermal conductivity of approximately 0.013 W/m·K. In daylighting and solar energy applications, due to their transparent nature, aerogels are applicable. For this purpose, research on the development of highly insulating aerogel windows has been conducted over the last decade. In one European Union project named HILIT,3) monolithic aerogel-based windows were developed with a combination of vacuum glazing technology and the application of 1 to 10 mbar pressure. The prepared 13.5 mm aerogel glazing showed an overall heat loss coefficient Uwindow of 0.66 W/m2·K and solar transmittance TSOL of more than 0.85. Also, to allow for high thickness of the aerogel glazing, the heat loss coefficient was decreased, while a solar transmittance was observed. Cabot Aerogel commercialized two aerogel products, NanogelTM and Okagel.136) In 30 and 60 mm samples, Okagel has thermal conductivity of 0.018 W/m·K and heat transmittance coefficients between 0.6 and 0.3 W/m2·K, respectively.
4.2. Cryogenic application
Flexible aerogel blanket development was started in 1993 by Aspen Aerogels and the NASA Kennedy Space Center.134) Aerogel blanket insulation has been utilized in a number of cryogenic applications, predominantly in certain portions of LNG service, which operates at −165°C. Flexible aerogel blankets provide insulation for piping and equipment, with a reduction in damage during transport; they can also protect LNG loading arms from mechanical damage due to ice falls. The natural flexibility of aerogel blankets make them useful for the flex joints required in cryogenic application. The nanoporous and insulating properties of aerogels have advanced the requirements of Passive Fire Protection (PFP) and acoustic properties for cryogenic applications. Flexible aerogel blankets, due to their simple installation and reduced need for qualified labor, are useful in the installation of cryogenics in remote locations.
In the case of cryogenic applications, thermal insulation is a serious issue because it is required to maintain low temperatures (90 K for liquid oxygen and 4.2 K for liquid helium). Recently, NASA has sponsored research on densified liquid oxygen and hydrogen for spacecraft propellants. This field demands storage containers with superinsulation materials composed of nothing but aerogels. Multi-layered insulation (MLI) is required for many cryogenic systems, which require a high vacuum for prime effectiveness. Hence, flexible aerogel insulations have the potential to provide insulation at different vacuum levels without any damage; these materials are also easy to install and to maintain.
4.3. Other thermal insulation applications of aerogels
The first aerogel was patented in 1932; 70 years later, aerogels were selected as thermal insulation for the NASA Mars rover mission, for insulation of the Mars rover and also used in space suits.6) Aerogels have already been used on the European Retrieval Carrier (EURECA) Satellite to capture cosmic dust particles and were used in Space Shuttle experiments.6) NASA used these aerogels in the STARDUST project to capture cometary dust particles.
Flexible aerogels can be used in the present generation of super-insulating clothing that keeps people warm in cold weather with lower weight than that of traditional thermal clothing; it is also used in tents and sleeping bags. In refrigerators, the storage capacity can be increased by using aerogels for insulation. Flexible aerogels are more efficient than existing insulating materials. Kanamori et al.7,52,54) developed flexible and transparent aerogels with low thermal conductivity; these are promising materials for cost-effective aerogels based on thermal superinsulators.
5. Summary and Outlook
In recent years, thermal insulation has become a key technology for energy storage. A new kind of thermal insulating material with combining low thermal conductivity, transparency, and flexibility has been urgently required in the present research field. Flexible and transparent silica aerogels are an emerging class of porous material that is playing an important role in thermal insulation applications such as buildings and cryogenic insulation. The hierarchical porous structure of these materials, along with the high flexibility and transparency, can be easily tuned by employing surfactants and by controlling the sol-gel parameters. The different materials introduced here, such as alkoxysilane, bridging precursors, and polymers, as well as the organic-inorganic hybrid structure, can be useful to achieve porous structures with a combination of transparency and mechanical flexibility.
Though there is the possibility of preparing transparent and flexible aerogels that employ bridging alkoxysilane, the high cost of these precursors militates against further improvement for commercialization of these materials for thermal superinsulation. Hence, more emphasis should be placed on novel flexible as well as transparent silica aerogels made from cheap and environmentally-friendly precursors.
Acknowledgments
This research was supported by the Basic Science Research Program, through the National Research Foundation of Korea (NRF), funded by the Ministry of Education (2015R1D1A1A02062229). Author V.G. Parale would like to thank the Brain Korea 21 (BK21) Project for its financial support in the form of a postdoctoral fellowship.