Enhanced Crystallization Behaviour and Microwave Dielectric Properties of 0.9CaMgSi2O6-0.1MgSiO3 Glass-Ceramics Doped with TiO2
Article information
Abstract
The dependence of the microwave dielectric properties of the glass-ceramic composite 0.9CaMgSi2O6-0.1MgSiO3 on the crystallization behaviour was investigated as functions of the TiO2 content and heat-treatment temperature. The crystallization behaviour of the specimens was evaluated via a combination of the Rietveld and reference-intensity ratio methods. For specimens with a TiO2 content of up to 1 wt.%, a monoclinic diopside phase was formed, whereas a secondary TiO2 phase was formed with further increases in the TiO2 content. The quality factor (Qf) of the specimens was strongly dependent on the degree of crystallization. The highest Qf value was obtained with a TiO2 content of 0.5 wt.%, which was improved by increasing the heat-treatment temperature. The dielectric constant (K) was affected by the size of the crystallites and the TiO2 content. The temperature coefficient of the resonant frequency (TCF) was nearly constant for all of the specimens, regardless of the TiO2 content or heat-treatment temperature.
1. Introduction
In recent years, the applicability of low-temperature cofired ceramics (LTCCs) to technology has been widely investigated to meet the demand for miniaturized and multifunctional electronic devices. The key properties required for LTCC-based substrate materials include a low dielectric constant (K), high quality factor (Qf), near-zero temperature coefficient of the resonant frequency (TCF), and heat-treatment temperature that is much lower than the melting points of commonly used metallic electrodes (Ag, Au, or Cu).1)
CaMgSi2O6 and MgSiO3 (diopside and enstatite, respectively) glass-ceramic composites are good candidates for LTCC-based substrate materials because of the low heat-treatment temperature, low K, high Qf, and high mechanical strength of such systems.2) It has been reported that the dielectric properties of glass-ceramic composites can be affected by the density, porosity, crystalline phases formed, and degree of crystallization.3) According to our preliminary experiments, the glass-ceramic composite 0.9CaMgSi2O6-0.1MgSiO3 exhibits a high Qf value (36,000 GHz) when a high degree of crystallization is achieved.2) Therefore, improving the degree of crystallization of 0.9CaMgSi2O6-0.1MgSiO3 is the most important factor for enhancing the Qf value.
Various methods have been developed to improve the crystallization behaviour of glass-ceramic composites. One such method is controlling the heat-treatment temperature. 2,4,5) Generally, glass-ceramic composites that are heat-treated at the optimum temperature exhibit enhanced crystallinity. 5)
Another method for improving the crystallization of glass-ceramic composites is the addition of nucleation agents. With the addition of a nucleation agent, the crystallization of the glass can occur at both the surface and bulk, which can lead to an increase in the degree of crystallization; Cr2O3 and TiO2 have been widely investigated as nucleation agents for the CaMgSi2O6 system.4,6–11)
Cr2O3 is commonly used as a nucleation agent for diopside glass. It has been reported that the addition of Cr2O3 leads to the formation of heterogeneous nucleation sites in diopside glass; this process causes crystallization via the formation of MgCr2O4 spinel domains.7) However, Cr ions can exist in two valence states: Cr3+ and Cr6+.8) It has been reported that Cr3+ is miscible with diopside glass at high temperatures, while Cr6+ separates from the diopside glass at low temperatures because of the high field strength of the hexavalent state. These phenomena can degrade the degree of crystallization of a diopside glass.9) Therefore, Cr2O3 should be used alongside other nucleation agents for diopside glass, such as Fe2O3 or TiO2.4) On the other hand, TiO2 is a well-known nucleation agent for diopside glass. It can enter the glass as either a network modifier in the form of TiO6 at high temperatures, or as a network former in the form of TiO4 at low temperatures. Once TiO2 has been transformed into TiO6, it can decrease the viscosity of the glass, thereby increasing the mobility during crystallization. Therefore, the addition of TiO2 can enhance the crystallization behaviour of diopside glass.10,11) Thus, it is expected that the crystallinity of diopside glass can be improved by adding an optimum concentration of TiO2.
Even though many studies have investigated the effects of TiO2 on glass-ceramic composites, the two factors controlling the degree of crystallization have not been sufficiently clarified, i.e., the optimum concentration of TiO2 for glass-ceramic composites and the relationship between the dielectric properties and the degree of crystallization have not been reported. Moreover, the effects of the TiO2 content and the heat-treatment temperature on the crystallization behaviour of 0.9CaMgSi2O6-0.1MgSiO3 have not yet been investigated. Therefore, in this study, the microwave dielectric properties of 0.9CaMgSi2O6-0.1MgSiO3 were investigated as functions of the TiO2 content (0–6 wt.%) and the heat-treatment temperature. The crystallization behaviour of the specimens will be discussed based on how the degree of crystallization varies with the TiO2 content and the heat-treatment temperature.
2. Experimental Procedures
CaCO3 (99.9%), MgCO3 (99.9%), SiO2 (99.9%), and TiO2 (99.9%) powders were used as the precursor materials. The powders were mixed to achieve the desired composition of 0.9CaMgSi2O6-0.1MgSiO3, with the TiO2 content ranging from 0 to 6 wt.%; samples were ball-milled with ZrO2 balls for 24 h in ethanol. The mixed powders were then melted in Pt crucibles at 1500°C for 3 h and quenched in distilled water. Cullet of the samples was obtained by pulverizing the quenched products, which were then sieved through size-50 meshes. The glass frits were re-milled for 24 h and isostatically pressed into pellets at a pressure of 147 MPa. The pellets were then heat-treated at 850–950°C for 3 h in air.
The densities of the heat-treated specimens were measured via the Archimedes’ method. The crystalline phases and crystallization behaviours of the specimens were analysed with X-ray diffraction (XRD, D/Max-2500V/PC, RIGAKU, Japan). Scanning electron microscopy (SEM, JSM-6700F, JEOL, Japan) was used to evaluate the phase and crystallite size of the glass-ceramic specimens.
To evaluate the degree of crystallization of the specimens via a combination of Rietveld and reference-intensity ratio (RIR) methods, a sample of 10 wt.% a-Al2O3, which was annealed at 1500°C for 24 h to increase the crystallinity to 100%, was added to all of the samples to act as an internal standard.12,13) Rietveld refinements of the XRD patterns were performed with the Full-Prof program.14) The degree of crystallization of the samples was evaluated against the internal standard with Eq. (1):
where W, Wc, and Wstd are the weights of the specimen, crystalline component, and internal standard, respectively.12) The value of Wc/Wstd was calculated from the Rietveld quantitative analysis under the condition of Wc + Wstd = 1. The value of Wstd/W was determined by measuring the weights of the specimen and of the internal standard.13) The microwave dielectric properties were measured according to the method reported by Hakki and Coleman with the TE011 mode in the range of 11–13 GHz.15) The TCF values of the specimens were measured according to the cavity method over a temperature range of 25 – 80°C.16)
3. Results and Discussion
3.1. Physical Properties
The relative densities of the 0.9CaMgSi2O6-0.1MgSiO3 specimens with various TiO2 contents (0–6 wt.%) that were heat-treated at 850–950°C for 3 h are shown in Fig. 1. As the TiO2 content increases, the relative densities of all of the specimens decrease, but remain above 90% regardless of the TiO2 content.

Relative densities of the 0.9CaMgSi2O6-0.1MgSiO3 specimens with various TiO2 contents (0–6 wt.%) that were heat-treated at 850–950°C for 3 h.
Figure 2 provides SEM micrographs of the 0.9CaMgSi2O6-0.1MgSiO3 specimens with TiO2 contents of 0.5, 2, and 6 wt.% that were heat-treated at 850°C for 3 h. As the TiO2 content increases, the porosity increases because of the decreasing relative density, as shown in Fig. 1. These results can be attributed to the weak adhesion at the interfaces between the diopside (glass) and TiO2 (ceramic) phases, which arises from the degradation of pore elimination during the densification process and/or the difference between the thermal expansion coefficients of the diopside (8.40 × 106 °C−1 (crystal), 6.65 × 106 °C−1 (glass)17)) and TiO2 phases (8.9210−6°C1 18)). Considering the globular shape of the pores shown in Fig. 2, the degradation of pore elimination during the densification process is a major factor in the weak adhesion.

SEM micrographs of the 0.9CaMgSi2O6-0.1MgSiO3 specimens with various TiO2 contents (0.5–6 wt.%) that were heat-treated at 850–950 °C for 3 h: (a) 0.5, (b) 2, and (c) 6 wt.%.
Figure 3 provides XRD patterns of the 0.9CaMgSi2O6-0.1MgSiO3 specimens with various TiO2 contents (0–6 wt.%) that were heat-treated at 850°C for 3 h. The XRD patterns show that a single monoclinic diopside phase (CaMgSi2O6, JCPDS No. 71-1494) is detected for TiO2 contents of up to 1 wt.% (Fig. 3(b)). However, a secondary TiO2 phase (JCPDS No. 82-0514) becomes evident with further increases in the TiO2 content; this is confirmed by the XRD peak at 2q = 35.5° splitting into the diopside and TiO2 phases (Fig. 3(c)).

XRD patterns of the 0.9CaMgSi2O6-0.1MgSiO3 specimens with various TiO2 contents (0–6 wt.%) that were heat-treated at 850°C for 3 h: (a) 2q = 29–31°, (b) 2q = 15–80°, and (c) 2q = 34–37°.
To determine the crystallite size (L) of the specimens, the full width at half-maximum (FWHM) values of the XRD peaks were calculated with the Scherrer Equation (Eq. (2))19):
where L is the crystallite size, l is the wavelength of the X-ray radiation (l = 0.154 nm), B is the FWHM of the peak (in radians) after being corrected for instrumental broadening, and q is the Bragg angle. As the FWHM of an XRD peak decreases, the crystallite size increases. Fig. 3(a) shows that the specimens containing less than 1 wt.% of TiO2 have similar FWHM values, while those with TiO2 contents greater than 1 wt.% exhibit reduced FWHM values, which indicates that the crystallite size has increased.
3.2. Microwave Dielectric Properties
Figure 4 shows the relationship between the Qf values of the 0.9CaMgSi2O6-0.1MgSiO3 specimens and the TiO2 content (0 – 6 wt.%), as well as the effects of the heat-treatment temperature on the Qf values. The maximum Qf value for each temperature is achieved when the TiO2 content is 0.5 wt.%, and then the Qf values gradually decrease with further increases in the TiO2 content. In addition, the Qf values increase as the heat-treatment temperature increases from 850 to 950°C. Generally, the Qf value of a glass-ceramic composite is strongly dependent on the degree of crystallization, which can be improved by either adding a nucleation agent, such as TiO2, or increasing the heat-treatment temperature.
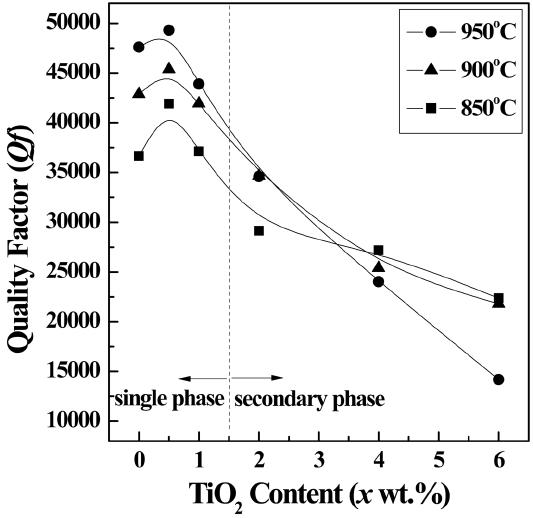
Quality factor (Qf) values for the 0.9CaMgSi2O6-0.1MgSiO3 specimens with various TiO2 contents (0–6 wt.%) that were heat-treated at 850–950°C for 3 h.
To evaluate the crystallinity of the 0.9CaMgSi2O6-0.1MgSiO3 specimens with various TiO2 contents (0–6 wt.%) that were subjected to heat treatments at different temperatures, the Rietveld-RIR method described in section 2 was used. The resulting Rietveld-refined patterns are shown in Fig. 5. The data points represent the measured intensities, the overlying solid lines are the calculated intensities, and the lower lines show the differences between the observed and calculated intensities. In addition, the short vertical bars indicate the Bragg reflections that correspond to the monoclinic diopside (top row), a-Al2O3 (middle row), and TiO2 (bottom row) phases. Table 1 shows that the R-factors, i.e. the goodness of fit (GoF), profile factor (Rp), weighted profile factor (Rwp), and expected weighted profile factor (Rexp), have low values, indicating that the Rietveld-refined results are reliable.
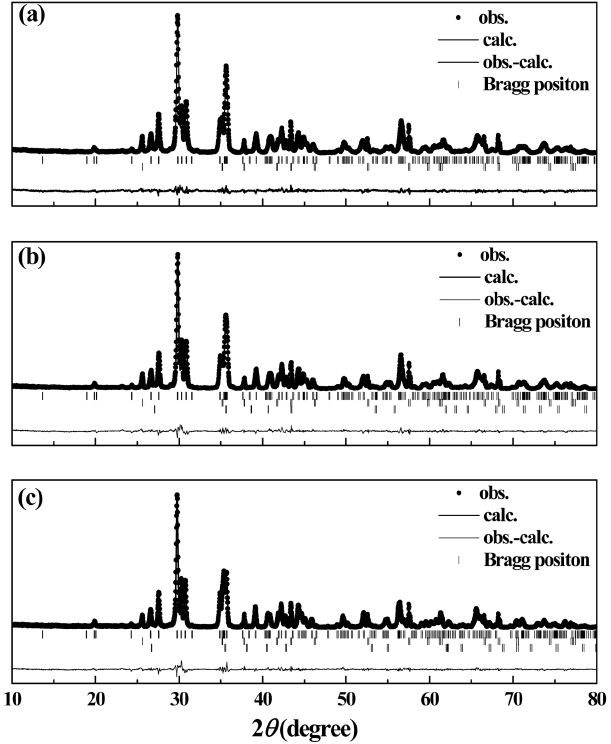
Rietveld-refined patterns of the 0.9CaMgSi2O6-0.1MgSiO3 specimens with various TiO2 contents (0.5–6 wt.%) that were heat-treated at 850–950°C for 3 h: (a) 0.5, (b) 2, and (c) 6 wt.%.

Results of the Rietveld-RIR Quantitative Analysis of the 0.9CaMgSi2O6-0.1MgSiO3 Specimens with Various TiO2 Contents (0–6 wt.%) that were Heat-Treated at 850–950°C for 3 h
Figure 6 shows the dependence of the Qf values on the degree of crystallization of the glass-ceramic composites. For the specimens composed of a single diopside phase (TiO2 content = 0–1 wt.%), the highest degree of crystallization is obtained with a TiO2 content of 0.5 wt.%; the crystallization then decreases and is nearly constant with further increases in the TiO2 content, as shown in Table. 1. In addition, the degree of crystallization of the specimens with TiO2 contents of 0.5 wt.% is enhanced as the heat-treatment temperature increases from 850 to 950°C. These tendencies are reflected in the trends of the Qf values of the specimens, as shown in Fig. 4. This is because single crystals always have smaller dielectric losses than the corresponding glass and, thus, high Qf values of the specimens are obtained when there is a high degree of crystallization.20) In addition, the Qf value of the specimen containing 0.5 wt.% of TiO2 and heat-treated at 950°C (Qf = 49,300 GHz) is similar to that of the CaMgSi2O6 ceramics sintered at 1290°C (Qf = 59,600 GHz21)), but the dielectric properties are not highly degraded because of the lower heat-treatment temperature.
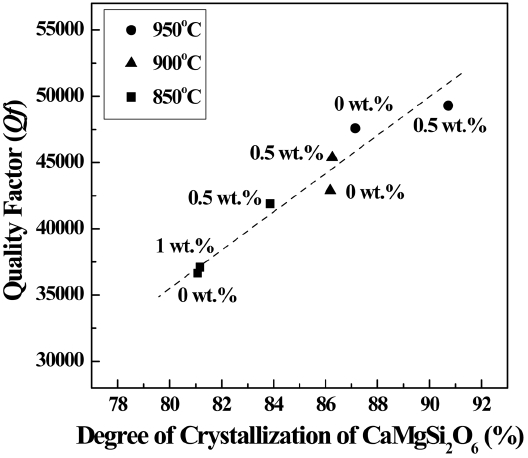
Dependence of the quality factor (Qf) on the degree of crystallization of the 0.9CaMgSi2O6-0.1MgSiO3 specimens with various TiO2 contents (0–1 wt.%) that were heat-treated at 850–950°C for 3 h.
Figure 7 shows the dependence of K on the crystallite size of the 0.9CaMgSi2O6-0.1MgSiO3 specimens with various TiO2 contents (0–6 wt.%) that were heat-treated at 850–950°C for 3 h. The crystallite sizes of the specimens are similar for TiO2 contents of up to 1 wt.%; then, the crystallite sizes increase from 30.8 to 37.0 nm with further increases in the TiO2 content, as shown in Fig. 3(a). The crystallite size increases with increasing TiO2 content because of the accelerated grain growth that is caused by heterogeneous nucleation. 22) For the specimens with TiO2 contents of 0.5 wt.%, the crystallite size also increases as the heat-treatment temperature increases from 850 to 950°C. It has been reported that K is dependent on the crystallite size of the specimens because of the reduced porosity of the interfaces between the grains.3,23,24) In addition, the increasing K values of the specimens could be caused by the higher intrinsic K of the secondary TiO2 phase (KTiO2 = 10025), while K = 6.98 for the 0.9CaMgSi2O6-0.1MgSiO3 phase).

Dependence of the dielectric constant (K) on the crystallite size of the 0.9CaMgSi2O6-0.1MgSiO3 specimens with various TiO2 contents (0 – 6 wt.%) that were heat-treated at 850–950°C for 3 h.
The TCF values of the 0.9CaMgSi2O6-0.1MgSiO3 specimens with various TiO2 contents (0–6 wt.%) that were heat-treated at 850–950°C for 3 h did not change significantly with the TiO2 content and/or heat-treatment temperature; the TCF values were approximately −20 ppm/°C for all of the compositions tested.
4. Conclusions
In this study, the effects of the TiO2 content (0–6 wt.%) and heat-treatment temperature on the microwave dielectric properties of 0.9CaMgSi2O6-0.1MgSiO3 glass-ceramic composites were investigated. The specimens with TiO2 contents of up to 1 wt.% and heat-treated at 850°C for 3 h exhibited a single phase of diopside (CaMgSi2O6), and then a secondary TiO2 phase became evident with further increases in the TiO2 content. For the specimens composed of a single diopside phase, the Qf values of these specimens were dependent on the degree of crystallization, which was affected by both the TiO2 content and the heat-treatment temperature; the highest degree of crystallization was achieved when the TiO2 content was 0.5 wt.%. Moreover, the degree of crystallization of the specimens with TiO2 contents of 0.5 wt.% was improved by increasing the heat-treatment temperature. The K values of the specimens were mainly affected by the size of the crystallites, while the TCF values did not change significantly. By adding 0.5 wt.% of TiO2 to the 0.9CaMgSi2O6-0.1MgSiO3 glass-ceramic composite (Qf = 49,300 GHz), the heat-treatment temperature was effectively lowered to 950°C.