1. Introduction
To overcome environmental issues such as global warming and air pollution, the development of sustainable energy technologies that are clean, affordable, and effectively infinite is required.1-3) Energy produced using renewable energy resources such as solar and wind energies is appealing; however, efficient energy storage systems (ESSs) are needed to compensate for the resulting intermittent energy production.1-5) To cope with the ever-increasing demands for the power sources to be used in grid-scale ESSs as well as portable electronic devices and electric vehicles (EVs), there have been many efforts to develop an efficient rechargeable battery system with high energy and power densities.1-12) Among known rechargeable batteries, Li-ion batteries (LIBs) offer the highest gravimetric and volumetric energy densities, which makes them the optimal choice for portable electronic devices and an appealing option for EVs and grid-scale ESSs.3-11) LIBs have dominated the portable electronic device market in the past two decades, and various types of EVs using LIB as power sources as well as prototype grid-scale ESSs have come to market during the past several years. In the pursuit of extended driving distances for EVs and lowered costs and long-lasting cycle performance for grid-scale ESSs, extensive efforts have been made to find potential candidates for next-generation cathode materials for LIBs.13-27)
Since Sony succeeded in commercializing the LIB system based on a LiCoO2 cathode and carbon anode, various types of cathode materials (e.g., layered, spinel, Li-rich, and polyanionic compounds) have been introduced and commercialized for LIBs. Among them, layered-type cathode materials, LiMO2 (M = Co, Mn, Ni, NixCoyMn1−x−y, or NixCoyAl1−x−y ), have received great attention for EVs and grid-scale ESSs because of their high gravimetric energy density (> 500 Wh kg−1) and good cycling performance.19,28-36) However, these electrode materials generally suffer from structural instability in the charged state or at high temperature due to transition metal migration and high oxygen activity, which can result in severe safety issues in practical applications. These safety concerns triggered the exploration of various polyanionic compounds (i.e., LiMPO4, Li2MPO4F, LiMSO4F, Li2MP2O7, where M = Fe, Mn, or Co) as cathode materials for LIBs because of the strong covalent bond of the polyanion, which stabilizes the oxygen ion within the crystal structure.23-25,37-57) However, these electrodes exhibit low energy density and low ionic and electronic conductivities, which limit their further development and application. Spinelstructured LiMn2O4 has been the focus of much attention because of its high structural stability, low cost, low toxicity, and relatively high energy density.58-67) LiMn2O4 has a facecentered cubic structure (space group Fd3m), with Mn and Li located at 16d octahedral and 8a tetrahedral sites, respectively. The spinel structure provides three-dimensional (3D) Li conduction pathways, which enable fast Li de/insertion upon electrochemical cycling. However, the electrochemical reaction of LiMn2O4 in Li-ion cells involves severe Jahn-Teller distortion and disproportionation due to existing Mn3+, resulting in poor cycle performance.64-67) Many previous studies have suggested that the electrochemical performance of LiMn2O4 can be improved by doping with different cations and anions.
Among doped LiMMn2−xO4 (M = Co, Fe, Cr, Cu, and Ni) materials, high-voltage spinel LiNi0.5Mn1.5O4, which utilizes the redox couples of Ni2+/Ni3+ and Ni3+/Ni4+, is considered a promising cathode for next-generation LIBs for EV and grid-scale ESS applications because of its high operating voltage of ~ 4.7 V (vs. Li+/Li), low cost, and good cycle and high-rate performances.68-83) However, several issues and challenges must be addressed in LiNi0.5Mn1.5O4: i) the unstable solid electrolyte interphase (SEI) formation at high operating voltage (vs. Li+/Li), ii) the Mn dissolution upon electrochemical cycling, and iii) the structural transition from the LiM2O4-type spinel structure to M3O4-type spinel and rock-salt structures at the particle surfaces. Many efforts have been made to enhance the electrochemical properties of LiNi0.5Mn1.5O4 by cation doping, applying electrolyte additives, controlling the particle size and morphology, controlling the degree of cation ordering, and surface modification.34,68-117) Among these solutions, the most efficient and simple way to prevent deleterious side reactions upon electrochemical cycling of LiNi0.5Mn1.5O4 is surface modification, which can enhance the overall capacity and cycle life.34,68-106) Efficient surface passivation prevents the direct contact of active particles with the electrolyte solution and suppresses the phase transition and side reactions at the particle surface, resulting in marked improvements in the electrochemical performance of electrode materials.
In this review, we will introduce the various surface modification techniques as well as the detailed synthesis methods used to stabilize the surface of the high-voltage spinel LiNi0.5Mn1.5O4 electrode material for LIBs. This review also covers the current state-of-the-art coating techniques based on metal oxides (i.e., ZnO, ZnAl2O4, Al2O3, TiO2, MgO, Fe2O3, CuO, RuO2, SiO2, SnO2, etc.), polyanionic compounds (i.e., Li4P2O7, Li3PO4, LiPO3, Li0.1B0.967PO4, LiFePO4, LiCoPO4, FePO4, Li2SiO3, LiAlSiO4, etc.), polymers (polyimide (PI), polyacrylate, polypyrrole (PPy), poly(ethyl α-cyanoacrylate)), and carbon-based materials (amorphous carbon, graphene oxide, carbon nanotubes (CNTs)). In addition, various characterization techniques used to evaluate coating layers on electrodes such as scanning electron microscopy (SEM), transmission electron microscopy (TEM), X-ray photoelectron spectroscopy (XPS), and Raman spectroscopy will be discussed. Finally, we will provide perspectives on the outlook of surface modification of LiNi0.5Mn1.5O4 materials.
2. Surface Modification Using Metal Oxide Compounds
Various metal oxides have received great attention as coating materials for spinel LiNi0.5Mn1.5O4. Several studies have reported on the use of ZnO coatings to protect the surface of LiNi0.5Mn1.5O4 against HF attack. Sun et al. reported that ZnO coating could improve the electrochemical properties of LiNi0.5Mn1.5O4.84) 1.5 wt% ZnO-coated LiNi0.5Mn1.5O4 delivered a charge capacity of 137 mAh g−1, and its capacity was maintained without any significant capacity degradation over 50 cycles at 55°C. Arrebola et al. also reported the effect of ZnO on nanosized spinel LiNi0.5Mn1.5O4. LiNi0.5Mn1.5O4 was easily wrapped by ZnO by mixing Zn(CH3COO)2·2H2O and LiNi0.5Mn1.5O4 in ethanol followed by heat treatment.85) The capacity of LiNi0.5Mn1.5O4/ZnO at the current rate of C/4 was ~ 128 mAh g−1 and was stably retained over 50 cycles. Sun et al. reported further enhancement of the electrochemical properties of LiNi0.5Mn1.5O4 through improvement of the electrical properties of ZnO by Al doping.86) The presence of the Al-doped ZnO coating was verified through TEM analyses, and the thickness of the coating was determined to be approximately 1 - 2 nm. The discharge capacity of the Aldoped ZnO-coated LiNi0.5Mn1.5O4 at 0.1C was ~ 128.9 mAh g−1 and, surprisingly, that at 10C was ~ 114.3 mAh g−1, which is ~ 77% of its theoretical capacity. Electrochemical impedance spectroscopy (EIS) analyses revealed that the Al-doped ZnO-coated LiNi0.5Mn1.5O4 had a lower charge transfer resistance than bare LiNi0.5Mn1.5O4 and ZnO-coated LiNi0.5Mn1.5O4, which might affect the improvement of the power capability of LiNi0.5Mn1.5O4. Furthermore, up to ~ 99% of the initial capacity of the Al-doped ZnO-coated LiNi0.5Mn1.5O4 was maintained for over 50 cycles at 50°C as well as at 25°C. Lee et al. modified the surface of LiNi0.5Mn1.5O4 through coating with ZnAl2O4, which provides low surface acidity, high thermal and chemical stability, and high mechanical resistance.87) For the ZnAl2O4 coating, LiNi0.5Mn1.5O4 particles were added to a solution in which Zn(NO3)2·6H2O and Al(NO3)3·9H2O were dissolved, and the solution was stirred at 80°C to form a gel. The gel was heated at 180°C for further esterification followed by annealing at 900°C for 12 h. TEM analysis revealed the formation of a coating layer with a thickness of less than 100 nm on the surface of the LiNi0.5Mn1.5O4 particles, and the presence of Zn and Al in the coating layer was confirmed through elemental analyses using TEM coupled with energy-dispersive X-ray spectroscopy (EDS). The capacity of the ZnAl2O4-coated LiNi0.5Mn1.5O4 at 1C was ~ 130 mAh g−1, which is ~ 88% of its theoretical capacity. In addition, the ZnAl2O4-coated LiNi0.5Mn1.5O4 delivered a capacity of ~ 97.3% of its initial capacity after 100 cycles at the current rate of 1C, whereas the pristine LiNi0.5Mn1.5O4 only provided ~ 89.0% retention of the initial discharge capacity. The thermal stability of the fully charged state of LiNi0.5Mn1.5O4 was also enhanced with the addition of the ZnAl2O4 coating. The exothermic reaction temperatures of the pristine cathode were ~ 181.8°C and ~ 267.1°C, whereas the exothermic reactions of the ZnAl2O4-coated LiNi0.5Mn1.5O4 occurred at ~ 192.1°C and 273.8°C, respectively.
Aluminum oxide (Al2O3) has also been used as a coating material for LiNi0.5Mn1.5O4. Wang et al. reported Al2O3-modified LiNi0.5Mn1.5O4 thin-film electrodes prepared by pulsed laser deposition (PLD).88) The LiNi0.5Mn1.5O4 thin-film electrodes contained grains smaller than 200 nm covered with extremely small Al2O3 particles. The Al2O3-modified LiNi0.5Mn1.5O4 exhibited enhanced cyclability with high coulombic efficiencies compared with pristine LiNi0.5Mn1.5O4, which means that the surface modification using Al2O3 resulted in improvement of the structural stability of LiNi0.5Mn1.5O4. Whereas the discharge capacity of the pristine LiNi0.5Mn1.5O4 at the 50th cycle was ~ 13.1 mAh g−1, which is ~ 10.6% of its initial capacity at 55°C, LiNi0.5Mn1.5O4 coated with 20-nm-thick Al2O3 exhibited ~ 89.3% capacity retention under the same conditions. Kim et al. also reported improvement of the electrochemical properties of LiNi0.5Mn1.5O4 by coating with Al2O3.89) They used atomic layer deposition (ALD) to achieve a homogenous coating of Al2O3 on the surface of LiNi0.5Mn1.5O4 particles. The deposition of an ~ 2-nm-thick amorphous layer on the particles was confirmed by TEM analysis. The SEI layer on the surface of the Al2O3-coated LiNi0.5Mn1.5O4 was much thinner and contained fewer organic species than that on the surface of the pristine LiNi0.5Mn1.5O4 because of the prevention of side reactions at high voltage provided by the Al2O3 coating. Thus, the Al2O3-coated LiNi0.5Mn1.5O4 exhibited significantly improved capacity retention compared with the pristine LiNi0.5Mn1.5O4. The coulombic efficiency of the Al2O3-coated LiNi0.5Mn1.5O4 after the 80th cycle was ~ 99.5%, whereas that of the pristine LiNi0.5Mn1.5O4 was 97.9%. Furthermore, it was verified that the Al2O3 coating could prevent the dissolution of transition metals in LiNi0.5Mn1.5O4. The Mn amount dissolved from the Al2O3-coated LiNi0.5Mn1.5O4 was ~ 6.6 times less than that from the pristine LiNi0.5Mn1.5O4, indicating the successful protection from HF attack provided by the Al2O3 coating. It has also been reported that a combination of nanostructuring and Al2O3 coating could improve the properties of LiNi0.5Mn1.5O4. Cho et al. prepared a LiNi0.5Mn1.5O4 nanowire electrode using the sol-gel-based template method. The average diameter and length of the LiNi0.5Mn1.5O4 nanowires were ~ 140 nm and ~ 13 μm, respectively.90) As observed in Fig. 1(a), the LiNi0.5Mn1.5O4 nanowires were uniformly coated by Al2O3 using ALD, and the thickness of the coating layer was ~ 1.2 nm. The ALD coating on the nanowire electrode enhanced the electrochemical properties of LiNi0.5Mn1.5O4. Fig. 1(b) shows that whereas the capacity of the uncoated LiNi0.5Mn1.5O4 nanowire was less than 10 mAh g−1 at a current rate of 1C and was significantly degraded over prolonged cycles, the Al2O3-coated LiNi0.5Mn1.5O4 nanowire exhibited a high coloumbic efficiency of ~ 98.8% over 25 cycles with a capacity at 1C of ~ 99 mAh g−1 (10 times that of the uncoated LiNi0.5Mn1.5O4 nanowire).
Titanium-based compounds have also been used as coatings for LiNi0.5Mn1.5O4. Tao et al. reported the improvement of the electrochemical stability of LiNi0.5Mn1.5O4 by surface modification with TiO2.91) The crystal structure of LiNi0.5Mn1.5O4 was not affected by the TiO2 coating, and no XRD peaks related to TiO2 were observed, which indicates the low loading amounts and thin layer of TiO2. Protective TiO2 layers were chemically coated on the surface of LiNi0.5Mn1.5O4 particles using a simple wet chemical method. The thickness of the coating layer of TiO2 was ~ 2 nm, and titanium and oxygen were homogenously distributed throughout the TiO2-coated LiNi0.5Mn1.5O4 particles. The TiO2-coated LiNi0.5Mn1.5O4 delivered a high discharge capacity of 74.5 mAh g−1 at 15C, which is a very fast current rate, and up to ~ 88.5% of its initial capacity was maintained over 500 cycles. In contrast, the capacity retention of the pristine LiNi0.5Mn1.5O4 was only ~ 33%. Li2TiO3 has also been considered as a coating material for LiNi0.5Mn1.5O4. Deng et al. reported the highly enhanced electrochemical performance of LiNi0.5Mn1.5O4 resulting from coating with Li2TiO3.92) As observed in Fig. 1(c), Li2TiO3 has received great attention because of its 3D Li diffusion path and great structural stability in organic electrolytes. The Li2TiO3 coating did not affect the structural deformation of LiNi0.5Mn1.5O4 or the formation of impurities. The thin and uniform Li2TiO3 coating on the surface of LiNi0.5Mn1.5O4 particles was verified by TEM coupled with elemental mapping. Elemental Ti was homogeneously distributed throughout the particles, and the thickness of the coating layer was ~ 8 nm. At a fast current rate of 5C, 3 wt% Li2TiO3-coated LiNi0.5Mn1.5O4 exhibited a discharge capacity of 95 mAh g−1, which is ~ 65% of its theoretical capacity, whereas the capacity of pristine LiNi0.5Mn1.5O4 was 70 mAh g−1 under the same conditions. Furthermore, 3 wt% Li2TiO3-coated LiNi0.5Mn1.5O4 exhibited better capacity retention than pristine LiNi0.5Mn1.5O4. As shown in Fig. 1(d), at 55°C, up to ~ 94.1% of the initial capacity of Li2TiO3-coated LiNi0.5Mn1.5O4 was maintained over 50 cycles. In contrast, the pristine LiNi0.5Mn1.5O4 only exhibited 77.1% capacity retention under the same conditions.
Alva et al. studied the surface modification of LiNi0.5Mn1.5O4 using MgO.93) Elemental Mg was primarily applied as an inhomogeneous MgO coating on the surface of LiNi0.5Mn1.5O4 particles via heat treatment at 500°C and was then doped into the crystal structure at the subsurface of the particles at 800°C. Using EDS elemental mapping coupled with SEM, the uniform encapsulation of each particle of LiNi0.5Mn1.5O4 by Mg was clearly verified. XPS analyses also supported the presence of Mg on the surface of the particles. At 50°C, the capacity of the Mg-coated LiNi0.5Mn1.5O4 was ~ 70 mAh g−1 at 5C, whereas that of pristine LiNi0.5Mn1.5O4 was ~ 50 mAh g−1.
Wang et al. reported the surface coating of Fe2O3 for LiNi0.5Mn1.5O4.94) The LiNi0.5Mn1.5O4 was modified by Fe2O3 through a simple chemical precipitation method using polyvinyl pyrrolidone polymer as a surfactant. The existence of elemental Fe in the Fe2O3-coated LiNi0.5Mn1.5O4 was confirmed through atomic absorption spectroscopy (AAS) and XPS. No Fe2O3 peaks were detected in the XRD pattern of the Fe2O3-coated LiNi0.5Mn1.5O4, which indicated that the Fe2O3 coating layer might be amorphous or that the amount of Fe2O3 was too small to be detected. Although the initial discharge capacity of the Fe2O3-coated LiNi0.5Mn1.5O4 was ~ 126 mAh g−1, which was slightly lower than that of pristine LiNi0.5Mn1.5O4 (~ 131 mAh g−1), the Fe2O3-coated LiNi0.5Mn1.5O4 exhibited better cycle performance than pristine LiNi0.5Mn1.5O4. Fe2O3-coated LiNi0.5Mn1.5O4 exhibited excellent capacity retention of ~ 98.6% at room temperature (RT) over 100 cycles and ~ 92% capacity retention at 50°C over 50 cycles, whereas the capacity retention of pristine LiNi0.5Mn1.5O4 was ~ 86.5% at RT and 79% at 50°C. Additionally, Fe2O3-coated LiNi0.5Mn1.5O4 also exhibited excellent power capability, delivering ~ 67% of its theoretical capacity at 10C.
CuO has also been considered as a potential coating material for LiNi0.5Mn1.5O4. Li et al. reported enhanced properties of LiNi0.5Mn1.5O4 resulting from CuO coating.95) For the CuO coating, pristine LiNi0.5Mn1.5O4 and Cu(H3COO)2·H2O were mixed in distilled water with vigorous stirring, and the mixture was heated at 500°C for 6 h in air. TEM analyses revealed the uniform coating of 3 wt% amorphous CuO on the surface of the LiNi0.5Mn1.5O4 particles with a coating thickness of ~ 3 nm. The CuO-coated LiNi0.5Mn1.5O4 exhibited great power capability compared with that of pristine LiNi0.5Mn1.5O4. The capacity of the 3 wt% CuO-coated LiNi0.5Mn1.5O4 was 98.7 mAh g−1 at 10 C, which was ~ 1.7 times higher than that of pristine LiNi0.5Mn1.5O4 and was stably retained over 100 cycles without any significant capacity degradation.
Coating of LiNi0.5Mn1.5O4 with RuO2 has been studied by several researchers because of the high electronic conductivity and catalytic functions for electrochemical oxidation processes of RuO2. Hong et al. reported a mechanism to improve the cycle performance of LiNi0.5Mn1.5O4 by RuO2 surface modification.96) The electrochemical properties of pristine LiNi0.5Mn1.5O4 and RuO2-modified LiNi0.5Mn1.5O4 were evaluated at various cut-off potentials ranging from 3.0 to 4.5 V. The specific capacity of pristine LiNi0.5Mn1.5O4 at a cut-off voltage of 3 V was severely degraded to 18.0 mAh g−1 after 1000 cycles, which is only 13.8% of its maximum specific capacity, with ~ 59 mAh g−1 retained at the cut-off voltage of 4.5 V over 1000 cycles, which represents ~ 50.9% of its maximum capacity. The cyclability of RuO2-modified LiNi0.5Mn1.5O4 was significantly improved when the cut-off potential was 4.5 V, with RuO2-modified LiNi0.5Mn1.5O4 delivering a specific capacity of 77.3 mAh g−1 after 1000 cycles, corresponding to ~ 70.1% of its maximum capacity. Jung et al. also reported on RuO2-coated LiNi0.5Mn1.5O4 prepared by a wet chemical route with heat treatment at 450°C.97) The existence of elemental Ru was identified through SEM elemental mapping. In addition, TEM analyses confirmed that the LiNi0.5Mn1.5O4 particles were coated by polycrystalline RuO2 with a coating thickness of ~ 10 nm. The cyclabilities of the pristine and RuO2-coated LiNi0.5Mn1.5O4 electrodes were evaluated through charge/discharge tests at 0.1C for the first two cycles and then at 0.5C for subsequent cycles. The capacity retention of RuO2-coated LiNi0.5Mn1.5O4 with a loading weight of 9 mg cm−2 was ~ 96.1%, which was higher than the ~ 85.7% capacity retention of pristine LiNi0.5Mn1.5O4 measured under the same test conditions.
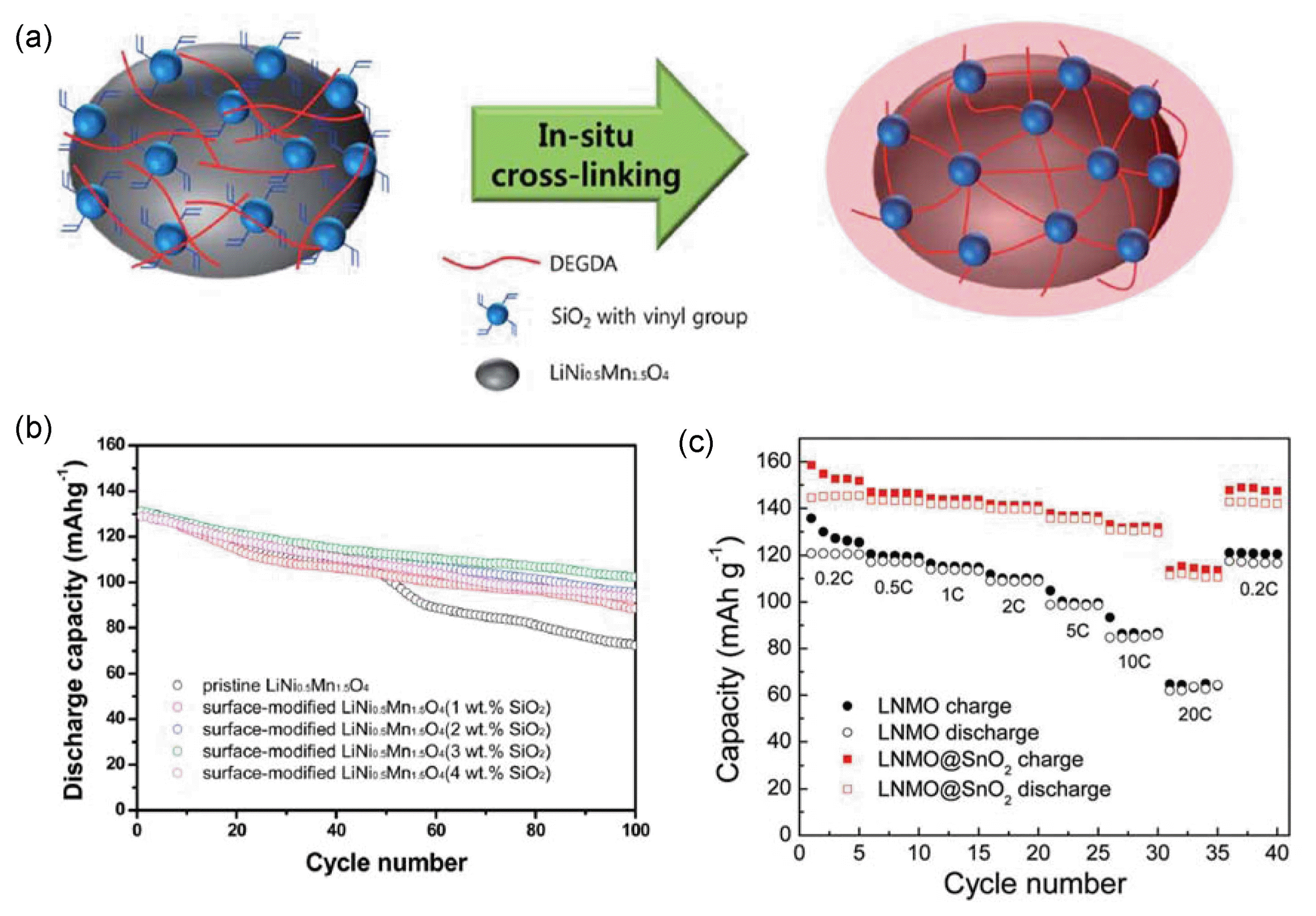
Several researchers have used SiO2 for improvement of the electrochemical performances of LiNi0.5Mn1.5O4. Fan et al. prepared SiO2-coated LiNi0.5Mn1.5O4.34) No significant structural change was observed with the SiO2 coating in the XRD analysis, and the TEM analyses confirmed that a porous nanostructured SiO2 layer with a thickness of ~ 30 nm wrapped the LiNi0.5Mn1.5O4 particles. The capacity retention of the pristine sample and the 0.5, 1.0, and 3.0 wt% SiO2-coated samples over 100 cycles was ~ 59%, 69%, 86%, and 87%, respectively, which indicated that the SiO2 coating improved the cycle stability of LiNi0.5Mn1.5O4. To verify the effect of the SiO2 coating on the electrochemical performance of LiNi0.5Mn1.5O4, the XPS spectra of the pristine and 1.0 wt% silica-coated samples measured at 55°C over 100 cycles were compared. The amount of LiF on the surface of the SiO2-coated sample was much lower than that on the surface of the pristine sample because of the reaction between SiO2 and HF. Because the LiF resulted in high impedance, preventing fast Li-ion diffusion, the SiO2-coated LiNi0.5Mn1.5O4 with the smaller LiF content exhibited lower polarization during charge/discharge as well as better cycle performance than the pristine LiNi0.5Mn1.5O4. Shin et al. prepared LiNi0.5Mn1.5O4 electrode materials encapsulated by a cross-linked composite polymer layer containing SiO2 nanoparticles through a chemical cross-linking reaction.98) Fig. 2(a) shows the synthesis process for SiO2-coated LiNi0.5Mn1.5O4. Whereas the pristine LiNi0.5Mn1.5O4 exhibited an initial coulombic efficiency of ~ 85.2%, the 3 wt% SiO2-coated LiNi0.5Mn1.5O4 delivered better coulombic efficiency of ~ 90.7%. As observed in Fig. 2(b), at 55°C, the pristine LiNi0.5Mn1.5O4 electrode suffered from severe capacity degradation, and its capacity after 100 cycles was ~ 55% of its initial capacity. However, the 3 wt% SiO2-coated LiNi0.5Mn1.5O4 electrode exhibited improved capacity retention of ~ 81% over 100 cycles. Furthermore, the pristine LiNi0.5Mn1.5O4 electrode experienced higher Mn and Ni dissolution, whereas no significant Mn and Ni dissolution from the SiO2-coated LiNi0.5Mn1.5O4 was detected. This finding indicates that the cross-linked composite polymer layer containing SiO2 particles provided effective protection from HF attack.
Ma et al. prepared SnO2-modified LiNi0.5Mn1.5O4 synthesized via a facile synthesis method.99) The average particle size of the SnO2-modified LiNi0.5Mn1.5O4 was smaller than that of LiNi0.5Mn1.5O4 because of the inhibition of crystal growth resulting from the formation of a thin SnO2 layer on the surface of the LiNi0.5Mn1.5O4 particles. EDS elemental mapping of the SnO2-modified LiNi0.5Mn1.5O4 revealed a homogeneous distribution of Ni, Mn, and Sn on the particles. As observed in Fig. 2(c), the capacity of the SnO2-modified LiNi0.5Mn1.5O4 at a current rate of 20C was ~ 112.2 mAh g−1, which is ~ 77.2% of its theoretical capacity, whereas the capacity of the pristine sample at 20C was ~ 64.3 mAh g−1. Over 500 cycles, the capacity of the SnO2-modified LiNi0.5Mn1.5O4 was maintained up to ~ 103 mAh g−1, representing retention of ~ 75% of its initial capacity, whereas that of the pristine sample decreased to 55 mAh g−1, which is only ~ 50% of its initial capacity. These electrochemical results indicated the improved power capability and greater cyclability of the SnO2-modified LiNi0.5Mn1.5O4 compared with those of the pristine sample.
Y2O3 coating has also been used for improvement of the electrochemical performance of LiNi0.5Mn1.5O4. Wen et al. prepared Y2O3-coated LiNi0.5Mn1.5O4 using a heterogeneous nucleation method.100) No significant structural differences were observed in the XRD patterns of the Y2O3-coated LiNi0.5Mn1.5O4 and pristine samples. The elemental mapping performed using SEM/EDS analyses revealed a homogenous distribution of Y on the surface of LiNi0.5Mn1.5O4. The thickness of the coating layer was determined to be ~ 6 - 8 nm based on TEM analyses. The Y2O3-coated LiNi0.5Mn1.5O4 exhibited better power capability than the pristine LiNi0.5Mn1.5O4. Whereas the capacity of the pristine LiNi0.5Mn1.5O4 was 56 mAh g−1 at 10C rate, the Y2O3-coated LiNi0.5Mn1.5O4 exhibited a remarkable capacity of 102 mAh g−1 at the same current rate, which corresponds to ~70% of its theoretical capacity despite the fast current rate. Furthermore, the capacity retention of the Y2O3-coated LiNi0.5Mn1.5O4 was ~ 91.6% over 100 cycles at 55°C, which is much greater than that of the pristine LiNi0.5Mn1.5O4, which was only ~ 77.9%.
Mou et al. reported the improved electrochemical performance of BiFeO3-coated LiNi0.5Mn1.5O4.101) The coating of 1.0 wt% BiFeO3 on the surface of the particles was shown to considerably enhance the power capability and cyclability of LiNi0.5Mn1.5O4. The 1.0 wt% BiFeO3-coated LiNi0.5Mn1.5O4 delivered discharge capacities of ~ 85.8 and ~ 74.8 mAh g−1 at current rates of 5C and 10C, respectively, whereas the pristine LiNi0.5Mn1.5O4 delivered discharge capacities of 77.5 and 60.9 mAh g−1, respectively, under the same conditions. The capacity retention of the 1.0 wt% BiFeO3-coated LiNi0.5Mn1.5O4 over 50 cycles at 55°C (~ 92.9%) was higher than that of the pristine LiNi0.5Mn1.5O4 (~ 80.3%).
Surface modification using a LiCoO2/Co3O4 composite has also been considered for the enhancement of the properties of LiNi0.5Mn1.5O4 because of the fast Li-ion transport into the LiCoO2 structure and the cubic spinel structure of Co3O4, which is similar to that of LiNi0.5Mn1.5O4. Qiao et al. prepared LiCoO2/Co3O4-modified LiNi0.5Mn1.5O4 using a simple liquid-phase process.102) No LiCoO2 or Co3O4 XRD peaks were detected, which indicates the absence of any structural deformation resulting from the surface modification. TEM analyses revealed that the thickness of the coating layer was ~ 10 - 60 nm, and the d-spacing values measured in the coating layer were ~ 0.200 and ~ 0.243 nm, corresponding to the (104) plane of LiCoO2 and (311) plane of Co3O4, respectively. The power capability and cyclability of LiNi0.5Mn1.5O4 were significantly improved after coating with LiCoO2/Co3O4. Up to ~ 85.5% of the theoretical capacity of the LiCoO2/Co3O4-modified LiNi0.5Mn1.5O4 was maintained at 5C, and its capacity over 200 cycles was ~ 97.8% of the maximal attainable capacity.
3. Surface Modification Using Polyanion Compounds
Various polyanion compounds have been used as coating materials for spinel LiNi0.5Mn1.5O4. Many researchers have reported on the coating effect of phosphate-based compounds for LiNi0.5Mn1.5O4. Chong et al. reported improvement of the electrochemical performance of Li4P2O7-coated LiNi0.5Mn1.5O4 resulting from the high ionic conductivity of Li4P2O7.103) The Li4P2O7-coated LiNi0.5Mn1.5O4 was synthesized using the conventional solid-state method, and using XRD analysis, the main phase of this compound was identified as LiNi0.5Mn1.5O4 with the cubic spinel structure with the coexistence of Li4P2O7. The TEM analyses confirmed that the thickness of the Li4P2O7 coating on the surface of LiNi0.5Mn1.5O4 was ~ 8 - 10 nm. The Li4P2O7-coated LiNi0.5Mn1.5O4 retained ~ 86.7% of its initial capacity at the current rate of 40C, whereas pristine LiNi0.5Mn1.5O4 only retained ~ 34.4% of its initial capacity at 40C. Furthermore, the cycle retention of LiNi0.5Mn1.5O4 was significantly improved by coating with Li4P2O7.
Li3PO4 has also been considered as a coating material for LiNi0.5Mn1.5O4. Konishi et al. reported the effect of Li3PO4 coating on LiNi0.5Mn1.5O4 using PLD.104) In the XRD analysis, besides for LiNi0.5Mn1.5O4, other peaks related to Li3PO4 were not detected, which indicates that the coating consisted of the amorphous phase of Li3PO4. X-ray reflectometer (XRR) analysis verified that the thickness of the Li3PO4 coating layer was ~ 0.7 - 3.6 nm. The Li3PO4-coated LiNi0.5Mn1.5O4 exhibited a larger specific capacity and reduced capacity degradation compared with pristine LiNi0.5Mn1.5O4. Chong et al. also prepared Li3PO4-coated LiNi0.5Mn1.5O4 as a stable high-voltage cathode material for LIBs.105) An ~ 5 - 6-nm-thick Li3PO4 coating was homogenously applied on the surface of LiNi0.5Mn1.5O4 using a solidstate synthesis method. The Li3PO4-coated LiNi0.5Mn1.5O4 exhibited much more stable cyclability than the uncoated LiNi0.5Mn1.5O4. The Li3PO4-coated LiNi0.5Mn1.5O4 retained 80% of its initial capacity (~ 122 mAh g−1) over 650 cycles. To further characterize the phase change after long cycles, dQ/dV plots of the Li3PO4-coated and uncoated LiNi0.5Mn1.5O4 at the first cycle and 200th cycle were compared. The Li3-PO4-coated LiNi0.5Mn1.5O4 did not experience a phase change, which indicates that the redox reactions of Ni2+/Ni3+ and Ni3+/Ni4+ were well retained even after 200 cycles.
LiPO3 has also been used as a coating material for LiNi0.5Mn1.5O4 because of its good ionic conductivity and chemical passivation properties. Chong et al. prepared LiNi0.5Mn1.5O4 coated with 1-nm-thick LiPO3 using the solidstate synthesis method.106) As shown in Fig. 3(a), the LiPO3 coating provided many advantages for improvement of the electrochemical performance of the LiNi0.5Mn1.5O4. The existence of the LiPO3 coating layer verified using XRD and Raman spectroscopy. Two Raman shifts were detected at 1077 and 1172 cm−1, representing the P-non-bridging oxygen in the LiPO3 phase. Fig. 3(b) shows that with increasing C rate, the LiPO3-coated LiNi0.5Mn1.5O4 exhibited significantly improved power capability compared with the pristine LiNi0.5Mn1.5O4. Furthermore, the LiPO3-coated LiNi0.5Mn1.5O4 exhibited 77% capacity retention at the fast current rate of 30C. These results indicate that the enhanced ionic conductivity resulting from the LiPO3 coating could efficiently lower the cell impedance and result in the superior power capability of LiNi0.5Mn1.5O4.
Li0.1B0.967PO4, which has been investigated as a solid electrolyte for LIBs because of its high Li ionic conductivity, was also applied as a coating material for LiNi0.5Mn1.5O4. Yang et al. prepared Li0.1B0.967PO4-coated and Cr-doped LiNi0.45Cr0.1Mn1.45O4 using the soft combustion reaction.68) No significant structural change was detected with the addition of the Li0.1B0.967PO4 coating, which indicated the presence of a separate layer of Li0.1B0.967PO4 on the surface of the LiNi0.45Cr0.1Mn1.45O4 particles. The Li0.1B0.967PO4-coated and Cr-doped LiNi0.45Cr0.1Mn1.45O4 exhibited outstanding cyclability with a capacity retention of ~ 85.4% over 400 cycles and excellent power capability with a capacity of ~ 90.7 mAh g−1 at 20C. These results indicate that the Li0.1B0.967PO4 coating layer provided a fast Li+ diffusion channel with low charge transfer resistance and served as a protective layer to achieve a more stable surface chemistry.
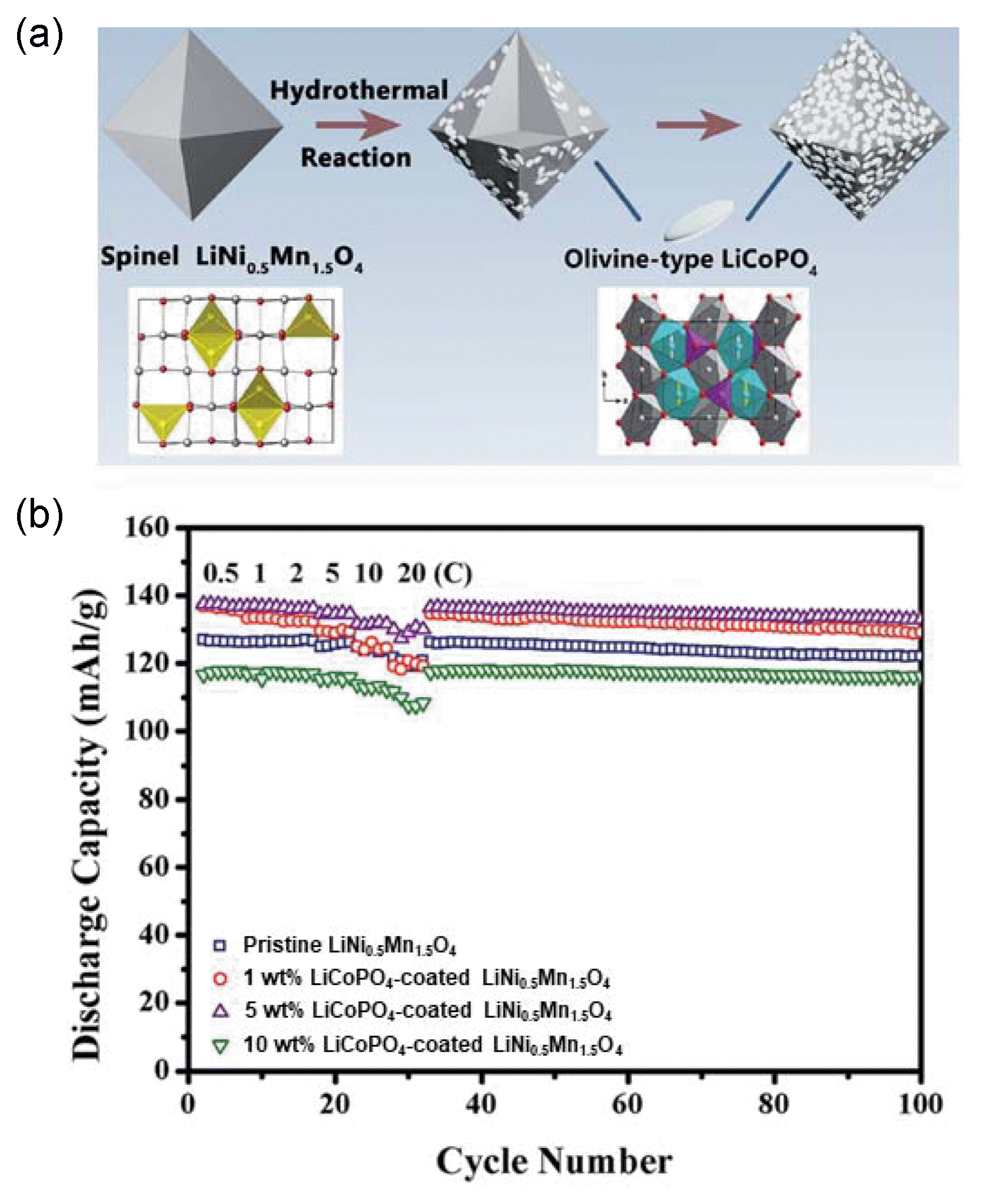
LiCoPO4 has also been applied as a coating material for spinel LiNi0.5Mn1.5O4 using a hydrothermal reaction synthesis method. Li et al. showed that the olivine LiCoPO4-shellcoated structure could significantly enhance the electrochemical performance by inducing the production of an appropriate amount of Mn3+.69) The synthesis process for the LiCoPO4-coated LiNi0.5Mn1.5O4 is illustrated in Fig. 4(a). The XRD peaks of the prepared samples were well indexed to the cubic spinel structure of the Fd3m space group with no impurity phase. It was identified through inductively coupled plasma-atomic emission spectrometry (ICP-AES) that increasing the coating amount of LiCoPO4 on the LiNi0.5Mn1.5O4 particles led to an increase in the Mn3+ content in the LiNi0.5Mn1.5O4. As observed in Fig. 4(b), the 5 wt% LiCoPO4-coated LiNi0.5Mn1.5O4 exhibited outstanding cyclability with 98.5% charge/discharge efficiency after 100 cycles and great power capability, delivering 130 mAh g−1 at a high current rate of 20C, compared with pristine LiNi0.5Mn1.5O4.
Jang et al. attempted to prepare LiFePO4-modified LiNi0.5Mn1.5O4 via a scalable sol-gel technique.70) No structural change occurred with the LiFePO4 coating, and the existence of LiFePO4 in the compound was verified through elemental mapping based on TEM measurements. At elevated temperature conditions of 55°C, the LiFePO4-modified LiNi0.5Mn1.5O4 delivered excellent cyclability with ~ 89% retention of its initial capacity after 50 cycles, whereas the pristine LiNi0.5Mn1.5O4 experienced severe capacity degradation under the same measurement conditions. The authors reported that this outstanding improvement of the electrochemical performances of LiNi0.5Mn1.5O4 resulted from the protective coating of LiFePO4, which prevented severe HF attack, because at high temperature, the reaction is prone to be accelerated compared with that at ambient conditions. EIS analysis revealed the lower charge-transfer resistance of the LiFePO4-modified LiNi0.5Mn1.5O4 than the pristine LiNi0.5Mn1.5O4 after 50 cycles at 55°C, demonstrating the benefit of the LiFePO4 coating for enhanced electrochemical activity of LiNi0.5Mn1.5O4 at high temperature.
Amorphous FePO4 was also considered as a coating material for LiNi0.5Mn1.5O4. Xiao et al. proposed the use of a novel ALD-derived ultrathin amorphous FePO4 coating.71) After applying the FePO4 coating using ALD, no peaks of FePO4 were detected in the XRD pattern because of the ultrathin and amorphous nature of the FePO4 phase. The intensity of the P 2p spectra decreased during ALD cycling, indicating that the amount of elemental P on the surface of LiNi0.5Mn1.5O4 increased with increasing number of ALD cycles. After 40 ALD cycles, no P 2p spectra was observed because of the surface saturation. HRTEM images revealed that the ultrathin FePO4 coating layer was ~ 2-nm thick, and the lattice fringe of ~ 0.24 nm distance was consistent with the (222) spacing of spinel LiNi0.5Mn1.5O4. The FePO4-coated LiNi0.5Mn1.5O4 prepared using 10 ALD cycles exhibited great electrochemical The performance, including a capacity of more than 80 mAh g−1 at 5C and retention of ~ 91.96% of the initial capacity after 100 cycles. Whereas pristine LiNi0.5Mn1.5O4 suffers from side reactions upon direct contact with the electrolyte, such as Mn dissolution and continuous electrolyte decomposition, the resistivity of the FePO4-coated LiNi0.5Mn1.5O4 prevented the direct contact of LiNi0.5Mn1.5O4 with the electrolyte, helping to prevent the reduction and dissolution of Mn ions caused by oxidation. Yi et al. also prepared FePO4-coated LiNi0.5Mn1.5O4 via a sol-gel method.72) XRD measurements indicated that the FePO4 coating did not change the structure of LiNi0.5Mn1.5O4. EDS mapping based on SEM imagining revealed the presence of Fe and P as well as Ni, Mn, and O, indicating the presence of FePO4 in the coated LiNi0.5Mn1.5O4. TEM analyses verified that LiNi0.5Mn1.5O4 particles were uniformly encapsulated by the ~ 1-nm-thick FePO4 layer, which indicates that FePO4 might have acted as an effective coating layer between the electrolyte and surface of LiNi0.5Mn1.5O4. In addition, 1 wt% FePO4-coated LiNi0.5Mn1.5O4 exhibited great power capability with a capacity of ~ 120 mAh g−1 at the fast current rate of 2C. After 80 cycles, the reversible discharge capacity of the 1 wt% FePO4-coated LiNi0.5Mn1.5O4 was ~ 117 mAh g−1, whereas that of the pristine sample was only ~ 50 mAh g−1.
Li2SiO3, a novel and typical Li+-ion conductor, has also been considered as a coating material for LiNi0.5Mn1.5O4. Deng et al. reported novel composites of (1−x)LiNi0.5Mn1.5O4· xLi2SiO3 prepared via a citric acid-assisted sol-gel method.73) The XRD peaks of the layer-structured Li2SiO3 and spinel LiNi0.5Mn1.5O4 were simultaneously detected in all the (1−x)LiNi0.5Mn1.5O4·xLi2SiO3 composites, indicating the coexistence of Li2SiO3 and LiNi0.5Mn1.5O4. The HRTEM images and selected area diffraction (SAED) patterns revealed that all the samples exhibited clear lattice fringes, indicating the good crystallinity of the materials. In addition, a thin layer was observed on the edge of the grains, which indicates the existence of amorphous Li2SiO3 on the surface of the LiNi0.5Mn1.5O4 particles. The Li2SiO3 coating could enhance the cyclic performance and power capability of the spinel LiNi0.5Mn1.5O4. The composite with x = 0.1 exhibited excellent power capability with a capacity of 107 mAh g−1 at the fast current rate of 2C and a capacity retention of ~ 85.3% over 50 cycles at 1C.
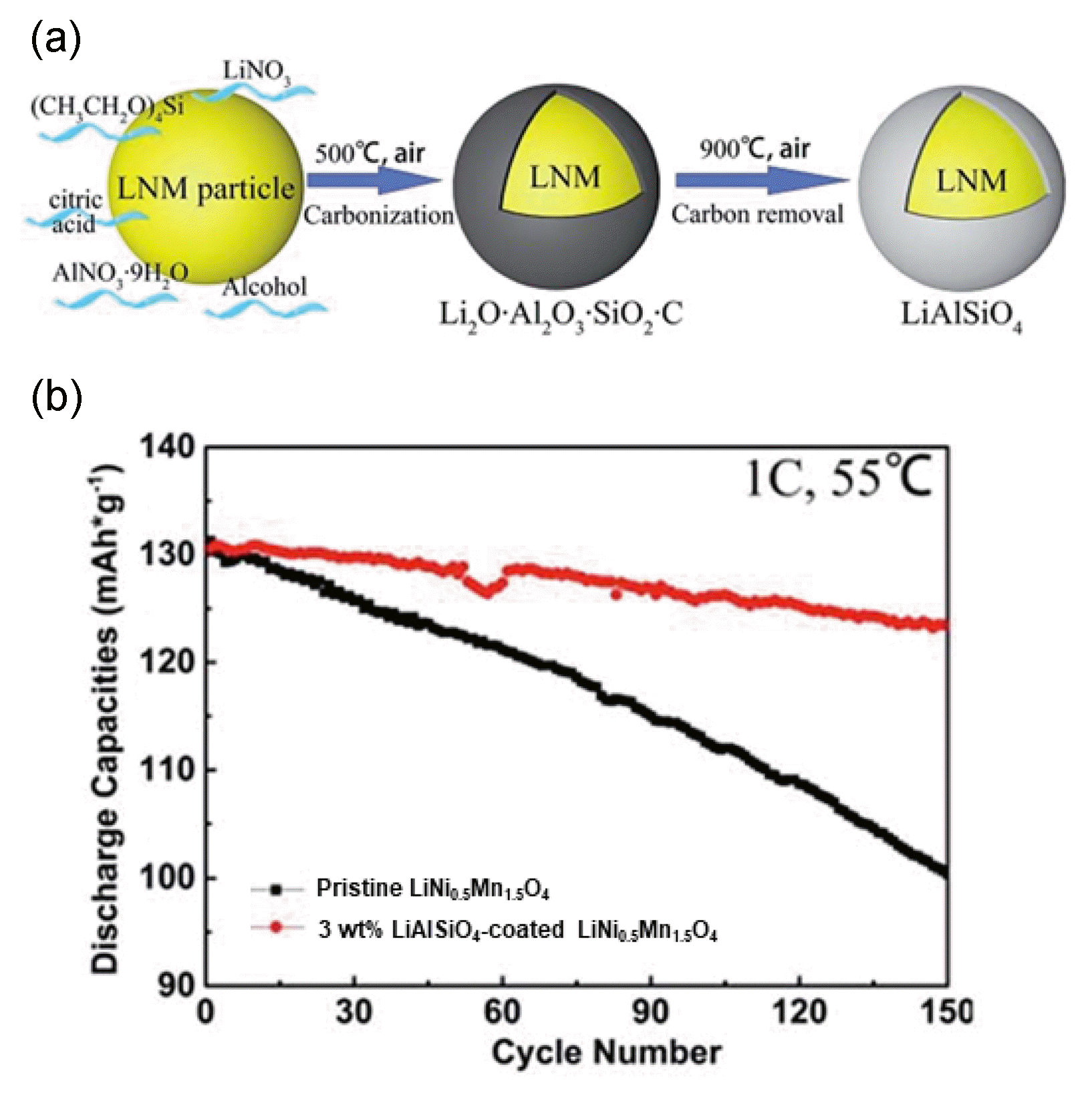
Deng et al. reported the coating of microporous LiAlSiO4 with good Li ionic conductivity on the surface of LiNi0.5Mn1.5O4 particles using a citric-acid-based sol-gel method.74) Fig. 5(a) presents a schematic illustration of the synthetic route used to produce the LiAlSiO4-coated LiNi0.5Mn1.5O4 particles. The XRD peaks of LiAlSiO4 were not detected for coatings containing 0 - 5 wt% LiAlSiO4. HRTEM analyses revealed that all of the samples had lattice fringes of ~ 0.48 nm, corresponding to the d-spacing of the (111) plane of spinel LiNi0.5Mn1.5O4. In addition, the samples except pristine LiNi0.5Mn1.5O4 were encircled by the coating layer of LiAl-SiO4. The 3 wt% and 5 wt% LiAlSiO4-coated LiNi0.5Mn1.5O4 displayed outstanding cyclability of 96.7% and 97.1% over 150 cycles at 25°C, respectively. Furthermore, the capacity of the 3 wt% LiAlSiO4-coated LiNi0.5Mn1.5O4 was maintained up to ~ 94.3% over 150 cycles at 55°C, whereas the pristine LiNi0.5Mn1.5O4 only exhibited capacity retention of ~ 76.5% under the same conditions (Fig. 5(b)).
4. Surface Modification Using Polymers
Cho et al. proposed coating LiNi0.5Mn1.5O4 particles with a polyimide (PI) gel polymer electrolyte (GPE) to prevent serious interfacial side reactions at high voltage, as shown in Fig. 6(a).75) The surface of the LiNi0.5Mn1.5O4 particles was encircled with PI via thermal curing of a four-component (pyromellitic dianhydride (PMDA)/biphenyl dianhydride (BPDA)/phenylenediamine (PDA)/oxydianiline (ODA)) polyamic acid. TEM measurements indicated that the LiNi0.5Mn1.5O4 particles were well encapsulated by an ~ 10-nm-thick PI layer. Fourier-transform infrared (FT-IR) spectroscopy peaks corresponding to the C=O bond of the PI layer were observed at 1723 cm−1, whereas the peak of the C=O bond of the pristine polyamic acid appears at 1650 cm−1. This shift of the C=O bond indicated that the polyamic acid was successfully imidized to form the polyimide wrapping the surface of the LiNi0.5Mn1.5O4 particles. No substantial differences were detected in the XRD patterns of the PI-coated and pristine samples. Compared with the pristine LiNi0.5Mn1.5O4, the PI-coated LiNi0.5Mn1.5O4 exhibited significantly improved cyclability. Up to ~ 120 mAh g−1 of the capacity of the PI-coated LiNi0.5Mn1.5O4 was retained after 50 cycles, whereas that of the pristine LiNi0.5Mn1.5O4 was only ~ 98 mAh g−1 after 50 cycles. Pang et al. also reported enhanced electrochemical properties of LiNi0.5Mn1.5O4 with PI coating.76) The PI-coated LiNi0.5Mn1.5O4 was prepared via a co-precipitation method and exhibited a spinel structure with P4332 symmetry, indicating that no structural change occurred as a result of the coating process. The PI coating prevented severe capacity fade of LiNi0.5Mn1.5O4 at the high temperature of 55°C, and the capacity retention of the PIcoated LiNi0.5Mn1.5O4 over 180 cycles was ~ 85%, which indicates the excellent thermal stability, good chemical resistance, and mechanical properties of the PI.
A polyacrylate coating was also applied to improve the electrochemical properties of LiNi0.5Mn1.5O4. Zhang et al. prepared lithium polyacrylate (PAALi)-coated LiNi0.5Mn1.5O4.77) The LiNi0.5Mn1.5O4 particles were uniformly surrounded by a thin disordered 1% PAALi layer, unlike the pristine LiNi0.5Mn1.5O4 particles without any coating layers. The 1% PAALi-coated and pristine samples retained up to ~ 90.0% and ~ 78.2% of their initial capacities over 200 cycles at 1C, respectively, which indicates the highly enhanced electrochemical performances of LiNi0.5Mn1.5O4 resulting from the PAALi coating. Furthermore, SEM analysis of the cycled electrodes of the 1% PAALi-coated and pristine samples showed that PAALi could encourage the formation of a SEI layer, which was compatible between LiNi0.5Mn1.5O4 and the electrolyte.
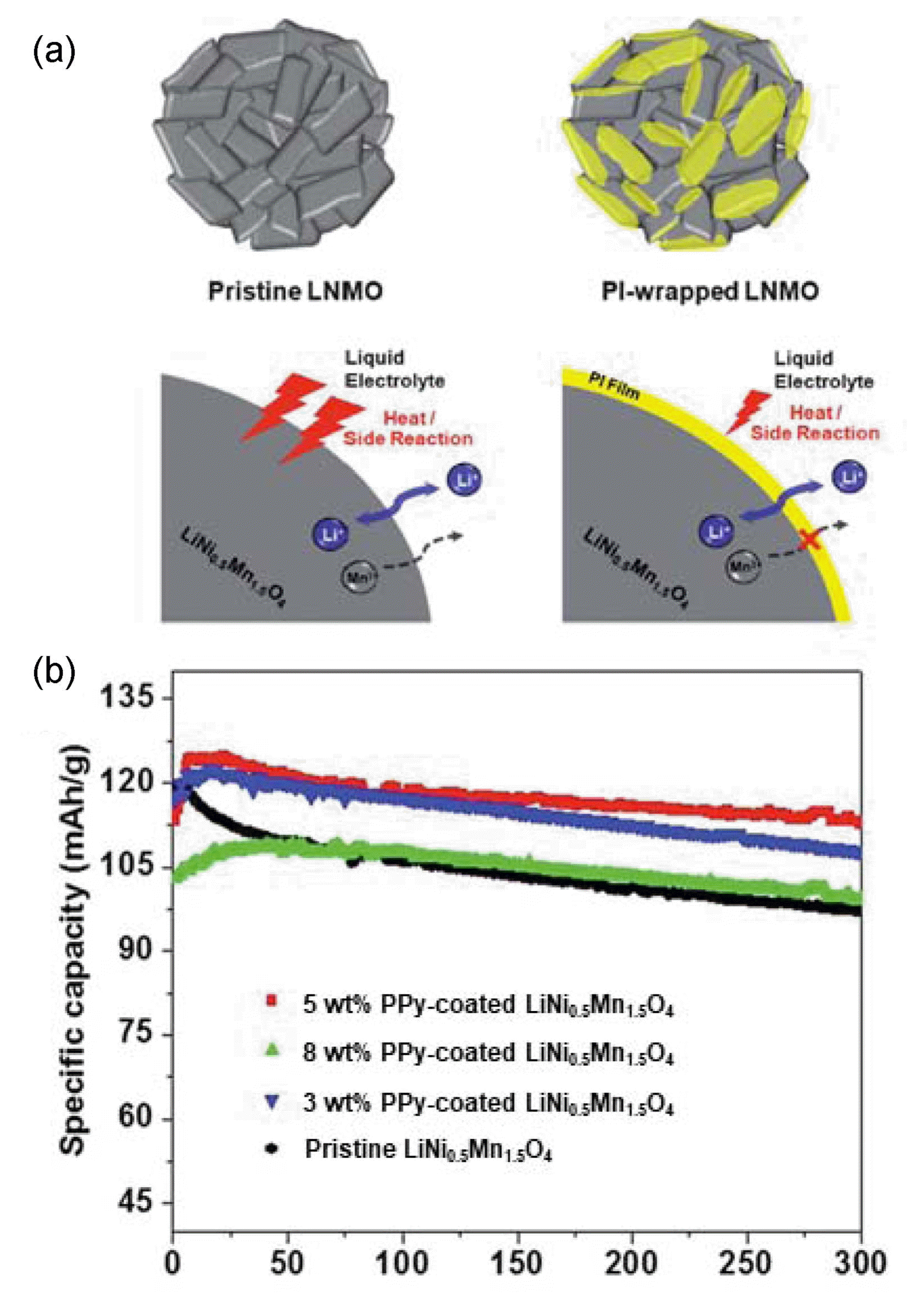
Conductive PPy coatings have also been applied to improve the electrochemical properties of LiNi0.5Mn1.5O4. Gao et al. prepared conductive PPy-coated LiNi0.5Mn1.5O4 composites using simple chemical oxidative polymerization in an aqueous solution.78) No structural differences were detected in the XRD patterns of the pristine and PPy-coated LiNi0.5Mn1.5O4 composites. SEM analysis indicated that the size of the LiNi0.5Mn1.5O4 particles was ~ 200 - 500 nm. TEM and EDS analyses revealed that the ~ 3-nm-thick porous PPy layer containing N well wrapped the LiNi0.5Mn1.5O4 particles. As observed in Fig. 6(b), whereas the pristine sample only exhibited capacity retention of 76.7% over 300 cycles, the 5% PPy-coated sample retained up to ~ 91% of its initial capacity after 300 cycles.
Poly(ethyl α-cyanoacrylate) (PECA) was also used as a coating material for LiNi0.5Mn1.5O4. Liu et al. prepared surface-modified LiNi0.5Mn1.5O4 particles via in situ polymerization of ethyl α-cyanoacrylate (ECA) to provide continuous surface coverage and a Li-ion transport channel.79) The XRD pattern of the PECA-coated LiNi0.5Mn1.5O4 was identical to that of pristine LiNi0.5Mn1.5O4, indicating that the polymer layer coating process did not affect the crystal structure of the LiNi0.5Mn1.5O4. The LiNi0.5Mn1.5O4 particles with a particle size of ~ 100 nm were encircled by an ~ 10-nm-thick PECA layer. Whereas the pristine LiNi0.5Mn1.5O4 delivered ~ 74.7% retention of its initial capacity over 100 cycles, the PECA-coated LiNi0.5Mn1.5O4 retained up to ~ 92% of its initial capacity under the same conditions. The polarization of the PECA-coated LiNi0.5Mn1.5O4 after 100 cycles was smaller than that of the pristine LiNi0.5Mn1.5O4, which demonstrates the effectiveness of the PECA layer in preventing the formation of the interface layer that hindered the charge transport at the interface between LiNi0.5Mn1.5O4 and the electrolyte.
5. Surface Modification Using Carbon-Based Materials
Carbon-based materials are known as excellent coating materials for electrode materials for LIBs because of their high electronic conductivity. Yang et al. reported enhanced electrochemical performance of carbon-coated LiNi0.5Mn1.5O4 prepared by a sol-gel method.80) The XRD pattern of the carbon-coated sample was characteristic of spinel LiNi0.5Mn1.5O4, and its particles were homogeneously wrapped by the carbon. The capacity of the carbon-coated LiNi0.5Mn1.5O4 modified with the addition of 1 wt% sucrose (sample c) remained up to ~ 130 mAh g−1 at 1 C and ~ 114 mAh g−1 at 5C. The carbon-coated sample also exhibited a high capacity retention of 92% after 100 cycles.
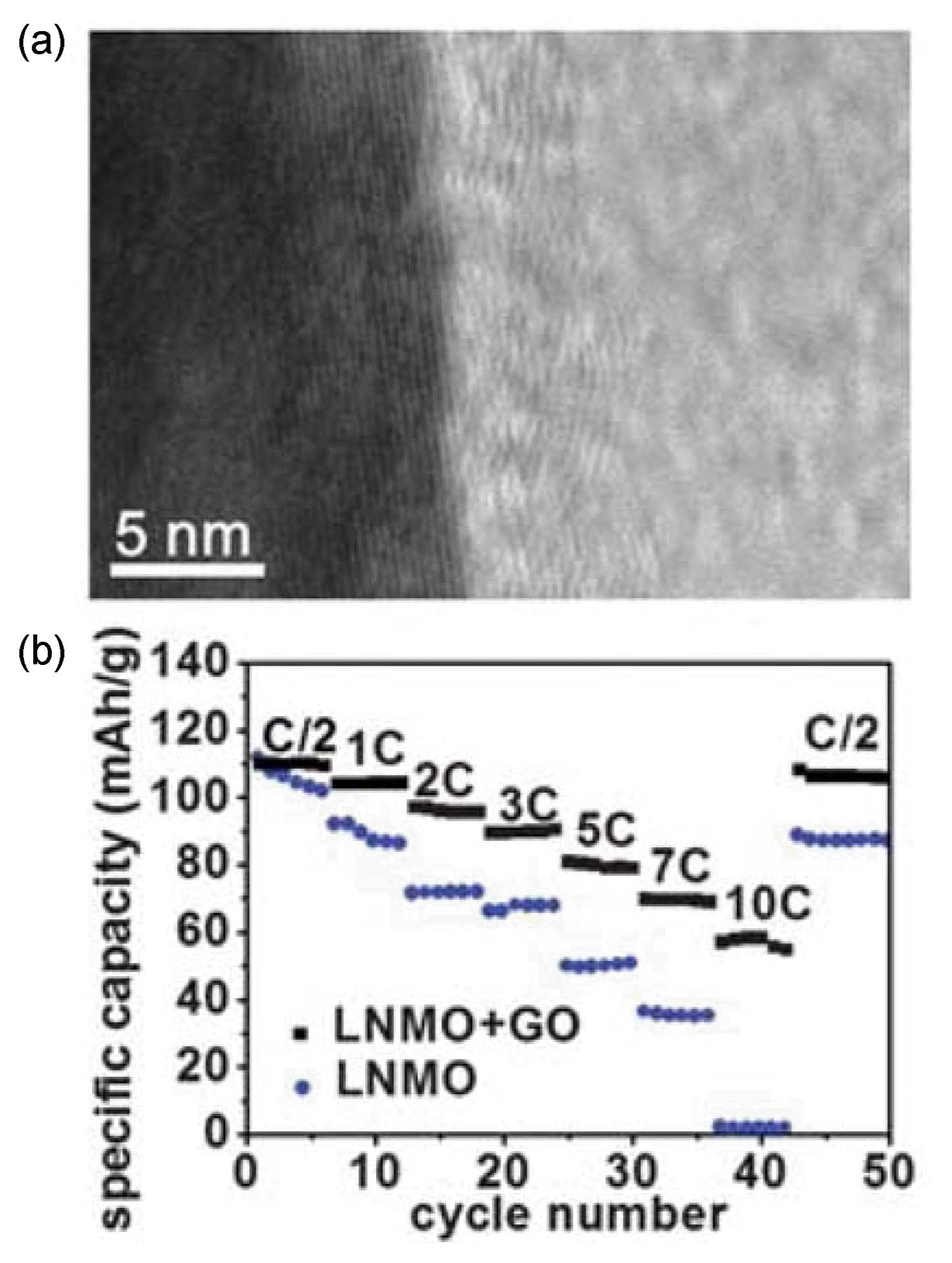
Graphene oxide was also applied as a coating material for LiNi0.5Mn1.5O4. Fang et al. synthesized graphene-oxidecoated LiNi0.5Mn1.5O4 through simple mixing and annealing.81) The graphene-oxide-coated sample had a pure spinel crystal structure without any contamination, and the existence of graphene oxide was verified through elemental mapping based on SEM. As shown in Fig. 7(a), TEM analyses revealed that graphene oxide with a thickness of ~ 5 nm was coated on the outer surface of LiNi0.5Mn1.5O4. The graphene-oxide-coated LiNi0.5Mn1.5O4 at large current densities of 5C, 7C, and 10C retained up to ~ 77%, ~ 66%, and ~ 56% of the capacity measured at 1C (Fig. 7(b)), and its capacity after 1000 cycles was ~ 61% of its initial capacity, indicating the enhanced electrochemical performances of LiNi0.5Mn1.5O4 resulting from the graphene-oxide coating.
Wang et al. synthesized a carbon-coated LiNi0.5Mn1.5O4 composite via pyrolysis of polyethylene glycol-400 (PEG) at high temperature.82) The carbon coating did not affect the crystal structure of LiNi0.5Mn1.5O4. A distinct coating layer was identified on the surface of the LiNi0.5Mn1.5O4 particles through TEM observation. The Raman spectra of carbon in the carbon-coated LiNi0.5Mn1.5O4 composite clearly showed a disordered (D) band at 1366 cm−1 and the ordered graphite (G) band at 1575 cm−1, verifying the existence of carbon in the composite. Over 500 cycles, the carbon-coated LiNi0.5Mn1.5O4 maintained up to ~ 71% of its initial capacity, whereas the pristine LiNi0.5Mn1.5O4 only retained ~ 55% of its initial capacity.
Surface modification of LiNi0.5Mn1.5O4 using CNTs has also been attempted. Hwang et al. prepared CNT-coated LiNi0.5Mn1.5O4 and oxidized CNT (OCNT)-coated LiNi0.5Mn1.5O4 composites via a mechano-fusion method to prevent unfavorable carbothermal reduction.83) The D and G peaks were verified in each Raman spectra of the CNT-LiNi0.5Mn1.5O4 and OCNT-LiNi0.5Mn1.5O4, verifying the existence of CNT and OCNT in each composite. The structures of the two composites were identical to that of the pristine LiNi0.5Mn1.5O4 without any structural changes. TEM observation revealed that the ~ 10 - 15-nm thick CNT or OCNT layers encircled the surfaces of the LiNi0.5Mn1.5O4 particles. The CNT-coated LiNi0.5Mn1.5O4 and OCNT-coated LiNi0.5Mn1.5O4 delivered higher energy densities than the pristine LiNi0.5Mn1.5O4 until the power density reached ~ 1260 W kg−1 at 2C.
6. Summary and Perspectives
Because of environmental problems such as air pollution and the greenhouse effect, considerable efforts have been made to design eco-friendly EVs, and LIBs have been considered one of the most attractive energy storage systems (ESSs) for EVs. To apply LIBs to these large-scale ESSs, however, their energy density must be improved. Thus, the development of electrode materials with theoretically high energy densities and high operation voltages is one of the major issues for LIBs in the world. Spinel LiNi0.5Mn1.5O4 has received great attention as an attractive cathode material for LIBs because of its high operating voltage of ~ 4.7 V vs. Li+/Li based on the Ni2+/Ni4+ redox reaction and relatively high theoretical capacity of ~ 146.7 mAh g−1. Recent studies on spinel LiNi0.5Mn1.5O4 have focused on surface coating using various compounds such as metal oxides, polyanionbased compounds, polymers, and carbon-based materials to improve the power capability and cyclability of LiNi0.5Mn1.5O4.
The desired coating material should exhibit high stability to prevent side reactions between LiNi0.5Mn1.5O4 and the electrolyte as well as the HF attack accompanying Mn dissolution from LiNi0.5Mn1.5O4. Even though the cyclability of coated LiNi0.5Mn1.5O4 at room temperature is not significantly enhanced compared with that of pristine LiNi0.5Mn1.5O4, coated LiNi0.5Mn1.5O4 exhibits higher stability than pristine LiNi0.5Mn1.5O4 during high-temperature cycle tests, which activate the side reactions and HF attack, because of the existence of the buffer layer for LiNi0.5Mn1.5O4. Furthermore, coating of compounds containing Li ions in the structure or carbon-based materials simultaneously enables the achievement of great power capability and outstanding cyclability of LiNi0.5Mn1.5O4 because of the high ionic or electronic conductivity of the coating material as well as the encapsulation of the particle surfaces.
Various experimental processes have been applied to achieve uniform and homogenous coatings of LiNi0.5Mn1.5O4 particles. Many researchers have prepared coated LiNi0.5Mn1.5O4 via heat treatment after mixing the LiNi0.5Mn1.5O4 with a precursor containing the element desired in the solution base. Some researchers have attempted to apply deposition methods such as PLD or ALD to obtain more uniform and homogenous coatings on LiNi0.5Mn1.5O4. The thickness of the coating layer is controlled by modifying the amount of reactants or the reaction time, and the existence of the coating layer in the composite is verified using SEM, TEM, XPS, or Raman spectra. It has been confirmed that the coating process does not affect the structure of LiNi0.5Mn1.5O4 regardless of the type of coating material.
To apply LiNi0.5Mn1.5O4 as a cathode material for LIBs, more intensive research on the stability of electrolytes for LIBs is required despite numerous reports on the stable cyclability of LiNi0.5Mn1.5O4 over several hundred cycles. Previous studies on LiNi0.5Mn1.5O4 have generally used halfcell tests based on small-scale coin cells. However, for the practical application of LiNi0.5Mn1.5O4 in commercialized LIBs, it is essential to perform full-cell tests of LiNi0.5Mn1.5O4 based on large-scale pouch cells. Furthermore, various analyses of the modified electrolytes in the full-cell test should be performed to verify the stability of the LIBs at high operation voltage resulting from the redox reaction of LiNi0.5Mn1.5O4.