Sonber, Murthy, Sairam, and Kain: Sintering and Oxidation of GdB4 Synthesized by B4C Reduction Method
Abstract
Gadolinium tetraboride (GdB4) was synthesized by reduction of Gd2O3 using boron carbide in presence of carbon. Effect of temperature on product quality was investigated. Pure GdB4 powder was obtained in vacuum at 1500°C. Pressureless sintering experiments revealed that sintering takes place only above 1600°C. A maximum density of 77.1% of the theoretical value was obtained at 1800°C by pressureless sintering. Hot pressing resulted in 95.5% of theoretical density at the lower temperature of 1700°C under 35 MPa pressure. Hardness and fracture toughness of dense GdB4 were measured and found to be 21.4 GPa and 2.3 MPa·m1/2, respectively. After exposure to air at 900°C, the formation of a porous and non-protective oxide layer was observed.
Key words: GdB4, Synthesis, Hot pressing, Boride, Oxidation
1. Introduction
Rare earth borides are exceptional ceramics characterized by high melting point, high hardness, excellent thermal stability, low vapour pressure, high electrical and thermal conductivity and low thermal expansion coefficient. 1) Due to high neutron absorption cross section of boron, rare earth borides are candidate materials for neutron absorber applications in nuclear reactors. 2-4) They are good thermionic electron emitters due to their low work function, low vapour pressure, high brightness and long service life. 5-7) Though borides of Lanthanum (La) and Cerium (Ce) have been studied extensively, literature on Gadolinium boride has been minimal. The high neutron absorbing capacity of Gd and boron makes the resulting compound suitable for neutron absorber applications in nuclear reactors. In the Gd-B system, the GdB 4 phase is the most stable. Important properties of GdB 4 are listed in Table 1. Most of the literature on GdB 4 has been concerned with its physical properties. 8-10) Reports on synthesis and densification of GdB 4 have been very limited. 5,11,12) Lazorenko et al. 11) prepared GdB 4 by borothermic reduction of Gd 2O 3 at around 2000°C in vacuum. Itoh et al. 12) prepared GdB 4 and GdB 6 by decomposition of the boron cage compound Gd 2(B 10H 10) 3. Gadolinium tetraboride powder is not easily available. In the present study, a process has been developed to prepare GdB 4 powder. In this process, GdB 4 was synthesized by boron carbide reduction of Gd 2O 3 in the presence of carbon, as per Eq. (1). We have not come across any report on the synthesis of GdB 4 powder by boron carbide reduction method. This method is expected to be economical since the raw materials are Gd 2O 3, B 4C and carbon, which are comparatively less expensive than elemental Boron and boron cage compounds used in previous studies. 11,12)
In many applications, borides are required to be in the form of dense shapes. Sintering of borides is a challenging task because the high melting point and low self diffusivity of these materials make sintering difficult. 13-15) Moreover, boride powders are generally covered with surface oxide layers that decrease the surface energy of the particles. 13-15) In this study, pressureless sintering and hot pressing techniques were investigated for obtaining GdB 4 in the form of dense pellets. Many borides are susceptible to oxidation, which limits their application in high temperature oxidizing environments. In this study, an oxidation test of GdB4 was carried out to explore the possibility of using this material in high temperature oxidizing environments.
This study reports on the results of investigations of the synthesis, consolidation and oxidation behaviour of GdB4. To the best of authors’ knowledge, there is no published report on densification and oxidation of GdB4.
2. Experimental Procedure
2.1. Starting material
Gd 2O 3 (99.9% purity, supplied by M/s Advanced Technology Materials, Mumbai), B 4C (78.5% B, 19.5% C, < 1% O, 0.02% Fe, 0.02% Si; 5.34 μm particle median diameter; supplied by M/s Boron Carbide India) and petroleum coke (99.4% purity; 13.9 μm median particle diameter, supplied by M/s Assam Carbon, India) were used as starting materials. The starting materials were characterized by X-ray diffraction (XRD) analysis using Cu Kα (λ = 1.5404 Å) radiation; this method has a detection limit of 4 vol%. Fig. 1 presents the XRD patterns of the starting powders, and confirms that there is no impurity phase in the starting powders, within the detection limit of XRD.
2.2. Synthesis
For the synthesis of GdB4, weighed quantities of gadolinium oxide, boron carbide and carbon in stoichiometric ratios were mixed thoroughly in a motorized mortar and pestle. The powder mixture was then pelletized under a pressure of about 200 MPa to obtain green pellets of 12 mm diameter. The pellets were then placed in a graphite crucible and heated in an induction furnace at a fixed temperature between 1200 to 1500°C for dwelling period of 2 h under a dynamic vacuum of 2 × 10−5 mbar. The temperature of the charge was measured using a two-colour pyrometer with an accuracy of ± 20°C. After the dwelling period, the furnace was allowed to cool to room temperature under vacuum; then, the partially sintered pellets were taken out, crushed and ground to a fine size using a high energy tungsten carbide lined cup grinding mill.
Major phases of powders were identified by XRD using Cu Kα (λ = 1.5404 Å) radiation. Carbon and oxygen were measured by combustion method and by inert gas fusion (IGF) method, respectively. Particle morphology was examined by scanning electron microscope (SEM).
2.3. Densification
In the pressureless sintering (PS) experiments, GdB4 powder was cold compacted under a pressure of about 280 MPa to form pellets of 12 mm diameter and 5 mm height with a green density of ~ 62% of the theoretical density (ρth). These compacts were sintered at a temperature between 1200 to 1800°C under a dynamic vacuum of 2 × 10−5 mbar in an induction furnace for holding periods of 2 h. In the hot pressing experiments, fine powders were hot pressed under a vacuum of 2 × 10−5 mbar in a high density graphite die (17 mm hole) by applying 35 MPa pressure at 1700°C for 2 h. The densities of the pellets were measured by the Archimedes principle. The fracture surfaces of the dense pellets was examined by SEM. The samples were broken by hammering to obtain a fracture surface.
Densified samples were polished to a mirror finish using diamond powder of various grades from 15 to 0.25 μm in an auto polisher (laboforce-3, Struers). Microhardness was measured at a load of 100 g and dwell time of 10 sec. Indentation fracture toughness (K Ic) data were evaluated by crack length measurement of the crack pattern formed around the Vickers indents (using 10 kg load), adopting the model formulation proposed by Anstis et al.16) K IC = 0.016(E/H) 1/2P/c 3/2, where E is the Young’s modulus, H the Vickers’s hardness, P the applied indentation load, and c the half crack length.
2.4. Oxidation
Isothermal oxidation study of the dense GdB4 was carried out at a temperature of 900°C for different time intervals. A hot pressed pellet of diameter 17 mm was cut into thin slices of 3 mm thickness. All the surfaces of the cut sample were ground with emery papers (1/0, 2/0, 3/0, 4/0) and finally polished with diamond paste to achieve a 1 μm finish. Oxidation tests were conducted in a resistance heated furnace. In order to avoid oxidation during heating, the sample was directly inserted into the hot zone after the furnace had reached the set temperature. Sample was placed in an alumina crucible loaded inside the furnace. The sample was oxidized in air for different time intervals (4, 8, 16, 32) at the set temperature of 900°C. The sample was carefully weighed to an accuracy of 0.01 mg before and after exposure, to determine the weight change during the oxidation process. The morphology and nature of the oxide layer were established by observing the surface in a field emission scanning electron microscope (FE-SEM) with energy dispersive spectroscopy (EDS). The phases present on the oxidized surface were analysed by XRD.
3. Results and Discussion
3.1. Synthesis
Table 2 presents the processing parameters and results of synthesis experiments carried out in a vacuum induction furnace. At 1200 and 1300°C, weight loss noticed is about 5.7% and 16.8%, respectively, which values are lower than the theoretical weigh loss (17.3%), indicating that the reaction had started but did not complete. The product was analysed and found to contain Gd 2O 3, GdB 4 and GdB 6 phases. The presence of Gd 2O 3 confirms the incomplete reduction. Sample processed at 1400°C was observed to contain GdB 4 and GdB 6 phases. Only at a temperature of 1500°C was single phase GdB 4 obtained. The carbon and oxygen content of GdB 4 were measured and found to be 0.5 wt.% each.
Figure 2 presents the XRD patterns of the samples processed at different temperature. The XRD patterns reveal that the product obtained at 1500°C is pure GdB 4, whereas products obtained at lower temperatures also contain the Gd 2O 3 and GdB 6 phase. The GdB 6 phase could have formed via the following side reaction ( reaction (2)).
At higher temperatures, the reaction kinetics for reaction (1) was fast and all the Gd 2O 3 was converted to GdB 4. In the Gd-B system, GdB 4 is the most stable phase. Free energy calculations for reactions (1) and (2) could not be done as thermodynamic data for GdB 4 and GdB 6 are not available. Though the reaction starts at a lower temperature (< 1200°C), pure GdB4 is obtained only at 1500°C. At low temperature, the reaction kinetics is very slow and hence a high temperature is required for faster diffusion of the solid reactant atoms through the product that formed at the interface.
The scientific literature on the processing of GdB 4 is scant, which was the motivation for this work. A summary of past studies is provided in Table 3. 5,11,12) The borothermic reduction process involves the use of elemental boron, which is expensive and pyrophoric. Preparation of GdB 4 by boron cage compounds and metal hydrides requires one additional step, as these materials are not readily available. Handling of metal hydride is also difficult due to its pyrophoric nature. Preparation by reaction between Gd 2O 3, B 4C and carbon seems to be the best process as it involves readily available charge material and does not have any handling issues. The process is also suitable for large scale production of boride powder. In this study, single phase GdB 4 was obtained. Synthesis of other rare earth borides by this route has been reported in literature. Zaykoski et al. have prepared aYB 4 ceramic by this route at 1800°C in helium atmosphere. 17) Liu et al. 18) have reported that NdB 6 powder can be prepared by boron carbide reduction of Nd 2O 3 at 1500°C in vacuum. Xu et al. 19) have prepared LaB 6 by this route at 1650°C at atmospheric pressure. Sonber et al. have prepared LaB 6, EuB 6 and CeB 6 by this route. 13,20,21)
3.2. Densification and characterization
The particle size distribution of synthesized GdB 4 powder that was ground using a high energy vibratory cup mill is presented in Fig. 3, which shows a bimodal distribution of particles with peaks at 2.5 mm and 10 mm. Mean particle diameter was found to be 5.6 mm. An SEM image ( Fig. 4) of the powder shows particle agglomerates of 4 - 7 mm size. Results of pressureless sintering and hot pressing experiments are summarized in Table 4. Results of pressure less sintering at temperatures upto 1600°C have shown that the obtained density is close to the green density of 63%, and that no densification took place during sintering. At 1800°C, densification started and a density of 77.1% of the theoretical value was obtained. The presence of high porosity (~ 23%) after sintering at 1800°C indicated that the densification of GdB 4 by pressureless sintering is extremely difficult. Hot pressing of GdB 4 at 1700°C under 35 MPa pressure resulted in a density that was 95.5% of the theoretical density. The poor sinterability observed in monolithic GdB 4 is due to this material’s high refractoriness and low intrinsic self diffusivity, which are also observed in other borides. 13,14) Arabei et al. 15) have reported that the densification processes of hexaborides becomes activated at temperatures in the range of 1800 - 2000°C. Zaykoski et al. 17) have prepared fully dense YB 4 by hot pressing at 1800°C.
Figure 5 provides an SEM image of the fracture surfaces of the pressureless sintered samples. Porosity is present in all the samples. The image also shows that there is no grain growth in the sample processed at 1200 and 1400°C. Grain growth was observed in the sample processed at 1800°C. Fig. 6 shows the fractured surface of the hot pressed GdB 4; this surface shows a dense microstructure.
3.3. Property characterization of hot pressed GdB4
3.3.1. Mechanical Properties
The hardness of the dense GdB 4 sample (95.5% dense) was measured and found to be 21.4 GPa. In the literature, the hardness of GdB 4 was found to be 18.6 GPa. 1) The fracture toughness of the dense GdB 4 sample (95.5% dense) was found to be 2.3 MPa·m 1/2. In the literature, there are no data available on fracture toughness measurement of GdB 4.
3.3.2. Oxidation study
Figure 7 presents the specific weight gain vs. time plot during isothermal oxidation of GdB 4 in air at 900°C. The plot shows linear behavior of the weight gain with time, which is a signature of a non-protective oxide layer. Specific weight gain values of 3.27 mg/cm 2 and 52.66 mg/cm 2 were measured after 4 h and 32 h, respectively.
Figure 8 provides an SEM image of the oxidized surface after oxidation. This image shows the presence of a porous and non-protective oxide layer on the surface. Fig. 9 shows the typical EDS pattern of the oxide layer. This indicates the presence of gadolinium, oxygen and boron on the oxidized surface. Fig. 10 shows the XRD pattern of the oxidized surface, which indicates the presence of GdBO 3 only as a crystalline phase. GdBO 3 could have been formed by reaction (3) during oxidation.
Free energy of formation for reaction (3) could not be calculated because thermodynamic data for GdB 4 are not available. There has been no report on the oxidation behaviour of GdB 4. In one study, 17) pure YB 4 was found to show considerable oxidation above 1200°C, and also resulted in the formation of a borate phase (YBO 3), similar to the oxidation behaviour of GdB 4 shown in the present study.
4. Conclusions
Conclusions that can be obtained from the present study are summarized as follows.
GdB4 powder was synthesized by boron carbide reduction of Gd2O3 at 1500°C in vacuum. Pressureless sintering study of monolithic GdB4 revealed that there is no densification until 1600°C. It was possible to achieve a maximum density of 77.1% of the theoretical density at 1800°C. Full densification was achieved by using hot pressing of GdB4 powder at 1700°C. Hardness and fracture toughness of fully dense GdB4 was measured and found to be 21.4 GPa and 2.3 MPa·m1/2, respectively. Oxidation study of monolithic GdB4 revealed the formation of a non-protective, porous oxidized layer consisting of GdBO3 after isothermal oxidation testing at 900°C in an air atmosphere.
Fig. 1
XRD pattern of starting materials. 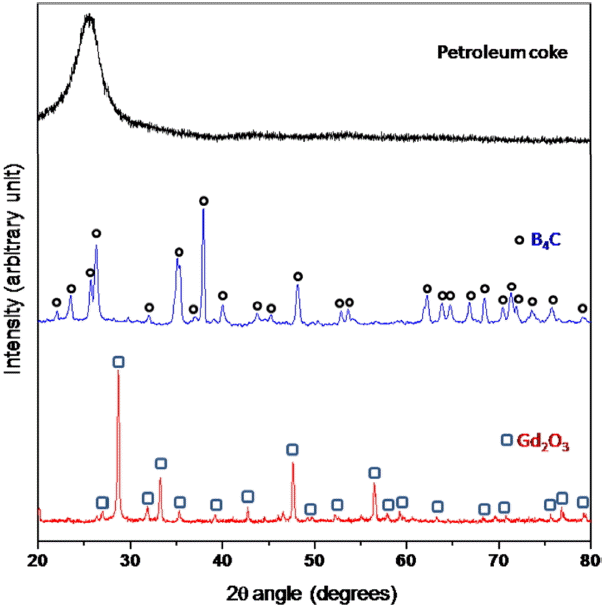
Fig. 2
XRD patterns of reaction product obtained at different temperatures. 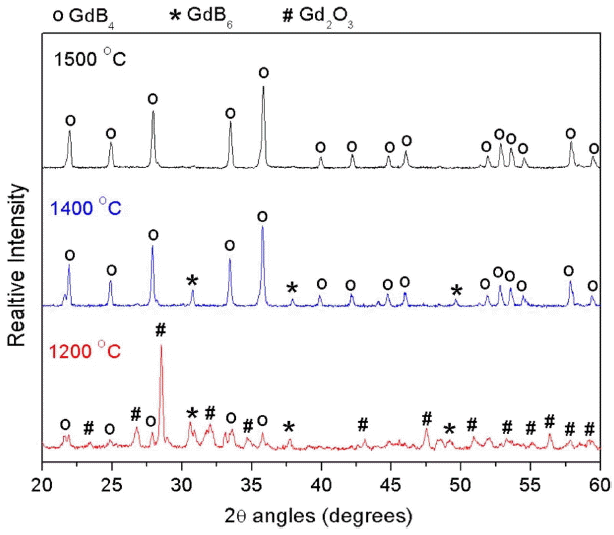
Fig. 3
Particle size distribution of GdB4 powder. 
Fig. 4
SEM image of GdB4 powder. 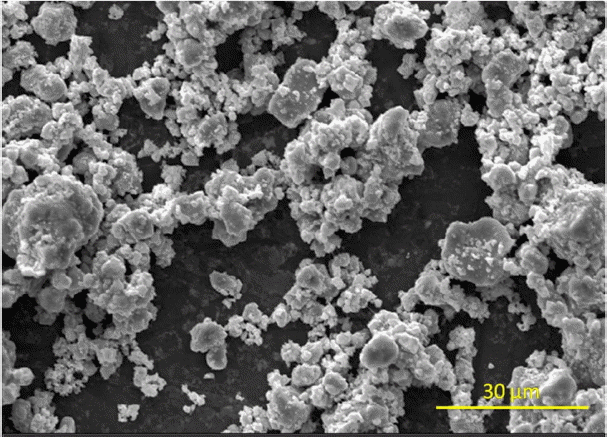
Fig. 5
SEM images showing fracture surfaces of GDB4 prepared by pressureless sintering at (a) 1200°C (b) 1400°C and (c) 1800°C. 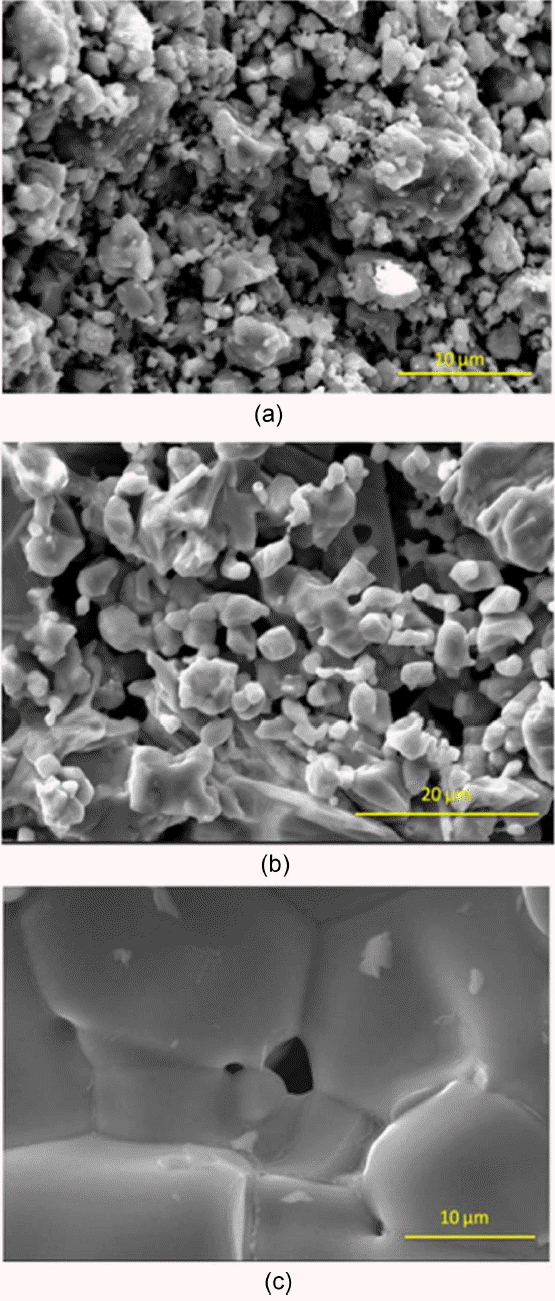
Fig. 6
Fracture surface of GdB4 hot pressed at 1700°C and 35 MPa. 
Fig. 7
Specific weight gain with time in GdB4 sample during oxidation at 900°C (line joining points are forvisual aid only). 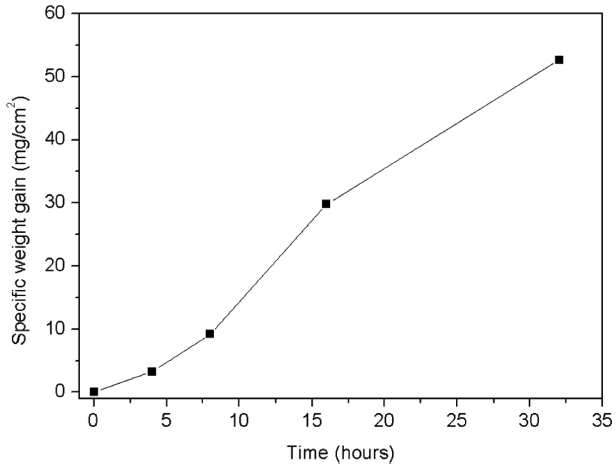
Fig. 8
SEM image of GdB4 oxidized at 900°C for 32 h. 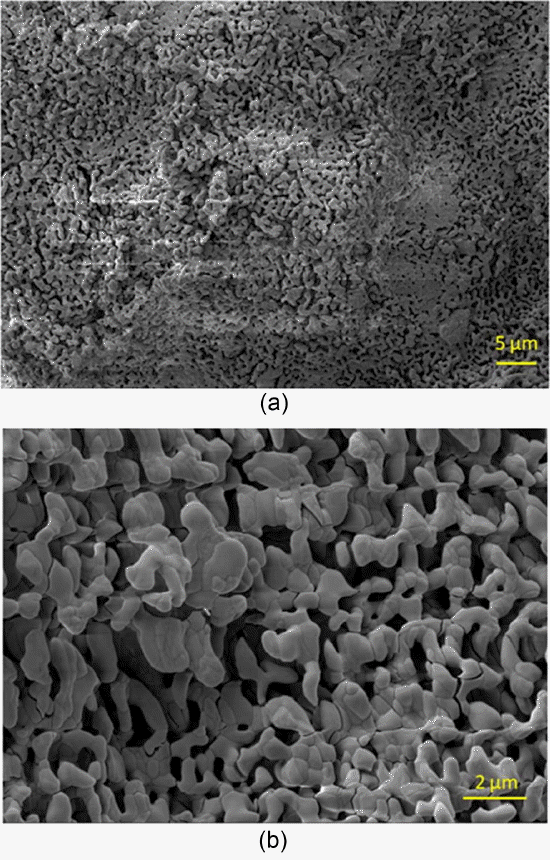
Fig. 9
Typical EDS pattern of oxidized surface of GdB4. 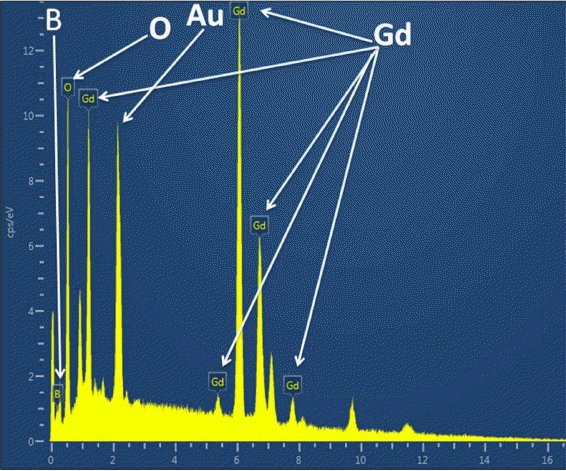
Fig. 10
XRD pattern of GdB4 oxidized at 900°C for 32 h. 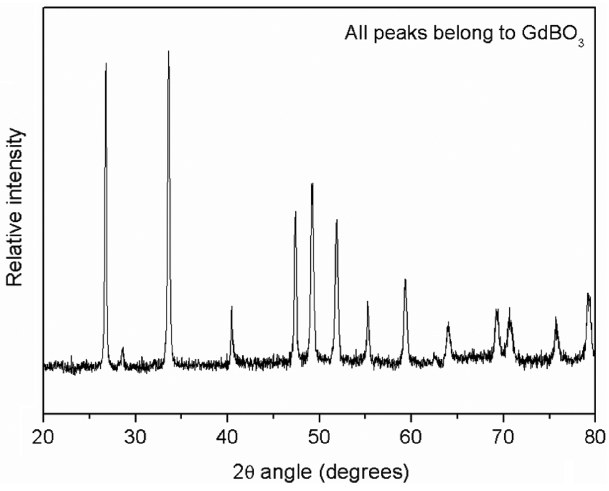
Table 1
Properties of Gadolinium Tetraboride 1)
|
Property |
GdB4
|
|
Crystal structure |
Tetragonal |
|
Density (gm/cc) |
6.47 |
|
Melting point (°C) |
2650 |
|
Thermal expansion coefficient (°C−1) |
7.0 × 10−6
|
|
Thermal conductivity (WM−1K−1) |
148.5 |
|
Hardness (GPa) |
18.6 |
Table 2
Results of GdB4 Synthesis Experiments
|
S. No. |
Temp. (°C) |
Weight loss (%) |
Phases present |
|
1 |
1200 |
5.7 |
Gd2O3, GdB4, GdB6
|
|
2 |
1300 |
16.8 |
Gd2O3, GdB4, GdB6
|
|
3 |
1400 |
18.2 |
GdB4, GdB6
|
|
4 |
1500 |
18.6 |
GdB4
|
Table 3
Summary of Studies on GdB4 Synthesis
|
S. No. |
Reactants |
Process Parameters |
Product quality |
Ref. |
|
1 |
Gd2O3 + B |
Temp.: 1997°C
Holding time: 1 h
Vacuum: 10−2 Pa (10−4 mbar) |
Single phase GdB4
|
5
|
|
2 |
Gd2(B10H10)3 + GdH2
|
Temp: 1200-1400°C
Atmosphere: Argon
Time: 3 h |
GdB4 and GdB6 phases |
12
|
|
3 |
Gd2O3 + B4C |
Temp.: 1470°C |
GdB6, GdB4
|
11
|
|
4 |
Gd2O3 + B4C + C |
Temp.: 1500°C
Vacuum: 10−5 mbar
Time: 2 h |
Single phase GdB4
|
Present Study |
Table 4
Results of Densification of GdB4
|
Technique |
Temperature (°C) |
Holding Time (h) |
Relative Density (%) |
|
Pressureless sintering |
1200 |
2 |
63.3 |
|
1400 |
2 |
65.2 |
|
1600 |
2 |
65.5 |
|
1800 |
2 |
77.1 |
|
|
Hot Pressing (35 MPa) |
1700 |
2 |
95.5 |
REFERENCES
1. ML. Bauccio, ASM Engineered Materials Reference Book; pp. 300-9 ASM International, USA, 1994.
2. EW. Hoyt, “Rare Earth Borides for Nuclear Applications,” pp. 287-89 In: Proceedings of the Second Conference on Rare Earth Research; Breach Science Publishers, New York1961.
3. P. Dunner, HJ. Heuvel, and M. Horle, “Absorber Materials for Control Rod Systems of Fast Breeder Reactors,” J Nucl Mater, 124 185-94 (1984).  4. B. Weidenbaum, EW. Hoyt, DL. Zimmerman, WV. Cummings, and KC. Antony, “Properties of Some High Temperature Control Materials,”; pp. 315-43 in Materials and Fuels for High Temperature Nuclear Energy Applications, In : Simnad MT, Zumwalt MR, editors, The M. I. T. Press, Massachusetts, 1962.
5. VI. Lazorenko, AP. Galasun, NI. Siman, AI. Dmitriev, and AV. Brodovoi, “Preparation and Properties of GdB4 Single Crystals,” Soviet Powder Metall Met Ceram, 29 [3] 224-27 (1990).  6. EK. Storms, and BA. Mueller, “Thermionic Emission and Atom Vaporization of the Gd-B System,” J Appl Phys, 52 [4] 2966-70 (1981).  7. DM. Goebel, Y. Hirooka, and TA. Sketchley, “Large-Area Lanthanum Hexaboride Electron Emitter,” Rev Sci Instrum, 56 [9] 1717-22 (1985).  8. JF. Rodríguez, JA. Blanco, K. Katsumata, A. Kikkawa, F. Iga, and S. Michimura, “Experimental Evidence of Non-collinear Magnetism in Gadolinium Tetraboride,” Phys Rev B, 72 [5] 052407(2005).  9. MT. Garland, JP. Wiff, J. Bauer, R. Guérin, and JY. Saillar, “The X-ray and Electronic Structures of GdB4,” Solid State Sci, 5 [5] 705-10 (2003).  10. A. Baranovskiy, and A. Grechnev, “Electronic Structure and Exchange Interactions in GdB4,” J Magn Magn Mater, 375 96-9 (2015).  11. W. Wu, J. Xu, X. Bian, G. Hu, S. Sun, and G. Tu, “Phase Constitution in Mixed Gd2O3 and B4C by Sintering at High Temperature,” J Rare Earths, 23 [3] 345-34 (2005).
12. H. Itoh, Y. Tsuzuki, T. Yogo, and S. Naka, “Synthesis of Cerium and Gadolinium Borides Using Boron Cage Compounds as a Boron Source,” Mater Res Bull, 22 [9] 1259-66 (1987).  13. JK. Sonber, K. Sairam, TSRC. Murthy, A. Nagraj, C. Subramanian, and RC. Hubli, “Synthesis, Densification and Oxidation Study of Lanthanum Hexaboride,” J Eur Ceram Soc, 34 [5] 1155-60 (2014).  14. JK. Sonber, and AK. Suri, “Syntheis and Densification of ZrB2: Review,” Adv Appl Ceram, 110 [6] 321-34 (2011).  15. BG. Arabei, EN. Shtrom, and YA. Lapitskii, “Characteristics of the Manufacturing Technology of Dense Parts from, and the Mechanical Properties of, Some Hexaborides of the Rare-Earth Metals,” Powder Metall Met Ceram, 3 [5] 406-9 (1964).  16. GR. Anstis, P. Chantikul, BR. Lawn, and DB. Marshall, “A Critical Evaluation of Indentation Techniques for Measuring Fracture Toughness: I, Direct Crack Measurements,” J Am Ceram Soc, 64 [9] 533-38 (1981).  17. JA. Zaykoski, MM. Opeka, LH. Smith, and IG. Talmy, “Synthesis and Characterization of YB4 Ceramics,” J Am Ceram Soc, 94 [11] 4059-65 (2011).  18. Y. Liu, WJ. Lu, JN. Qin, and D. Zhang, “A New Route for the Synthesis of NdB6 Powder from Nd2O3-B4C System,” J Alloys Compd, 431 [1] 337-41 (2007).  19. XH. Xu, HN. Xiao, WM. Guo, PZ. Gao, and SH. Peng, “Preparation and Reaction Mechanism of LaB6 Powder by Solid-State Reaction at Atmospheric Pressure,” J Inorg Mater, 26 417-21 (2011).  20. JK. Sonber, TSRC. Murthy, C. Subramanian, RC. Hubli, and AK. Suri, “Synthesis, Densification and Characterization of EuB6,” Int J Refract Met Hard Mater, 38 67-72 (2013).  21. JK. Sonber, TSRC. Murthy, K. Sairam, B. Paul, and JK. Chakravartty, “Effect of TiSi2 Addition on Densification of CeB6,” Ceram Int, 42 891-96 (2016). 
|
|





















