1. Introduction
China glaze refers to a glassy film layer formed on the matrix through high-temperature sintering process, and is applied to diversified household ceramics products such as household utensils, tiles, sanitary equipment, etc. The glaze exhibits diversified colors, gloss characteristics, textures, etc. depending on composition of the oxide used, sintering temperature and atmosphere, and may be classified accordingly. The reason for using the glazes is to prevent contamination or damages by preventing infiltration of liquids such as water into the interior through formation of uniform films with a thickness of 0.5 - 1 mm on the china surface. In addition, an increase in mechanical strength and chemical durability may be expected.1) By using the glaze, gloss and color may be generated on the product for decoration, or textures may be formed on the surface for expression. To materialize such characteristics of the glaze, control of reaction and differences between the glaze and the matrix is required as well as understanding on composition and sintering process of the glaze.
Stull has defined correlations between composition of the glaze and gloss characteristics by using Unity Molecular Formula (UMF) in 1912.2) UMF classifies oxides into alkali, neutral, and acidic oxides according to their roles. Alkali oxides playing the role of a flux include alkalis such as Na2O, K2O, Li2O (R2O), etc. and alkali earth oxides such as CaO, MgO, SrO, BaO, ZnO (RO), etc. Representative neutral oxides (R2O3) are Fe2O3 and Al2O3, while acidic oxides (RO2) include SiO2, and TiO2. When all oxides are converted to number of moles, and neutral and acidic oxides of these are divided by the sum of alkali oxides, a 2-dimensional composition table with acidic and neutral oxides as X-Y axes can be obtained. By displaying gloss characteristics and boundaries of the glazes on this composition table, Stull enabled prediction of gloss characteristics of a glaze from the composition of the glaze. Namely, by employing a chart between glaze composition and glaze characteristics constructed with the use of UMF method, glaze composition with the required gloss characteristics and sintering temperature were made to be easily determined.
In the present study, a correlation between glaze composition and characteristics (glossiness, surface roughness, crystalline phase, microstructure, hardness) was to be defined by using the method employed by Stull. The characteristics of a glaze analyzed here are design and technical elements which should be considered upon product development. A total of 35 types of glass composition (Al2O3: 0.2 - 0.6, SiO2: 1.5 - 4.5) were mixed by using UMF, and characteristics of a glaze were analyzed after sintering in oxidative (1250, 1315°C) and reductive (1250°C) atmospheres. From these results, changes in surface characteristics and mechanical properties as a function of glaze composition were checked, and differences in crystalline phases and microstructures observed in gloss characteristics region of the glaze were analyzed. Such results can not only be utilized as a guideline for glaze combinations but also facilitate development processes by direct application to design and production of china products.
2. Experimental Procedure
By using UMF, the ratio between alkali and alkali earth oxides was fixed to be 3 : 7, and 35 types of glaze were combined by varying the ratio between alumina (Al2O3) and silica (SiO2) in the range of 0.2 - 0.6 and 1.5 - 4.5, respectively, at the intervals of 0.1 and 0.5. Buyeo feldspar, Na2CO3, CaCO3, silica, kaolin, and alumina weighed according to composition ratios were mixed with distilled water in the ratio of 1 : 1 and then subjected to ball milling for 24 h.
Biscuit-fired white porcelain specimens of 50 × 50 mm in size had 35 types of glaze applied for glazing, followed by sintering under 3 types of conditions. To experiment for change in characteristics of the glaze as a function of sintering atmospheres and temperatures, sintering was conducted at 1250°C in oxidizing and reducing atmospheres, respectively, and oxidatively at 1315°C.
By measuring the reflectivity at 60° to the glaze surface, glossiness of the glaze was analyzed (Glossmeter, micro-TRI-gloss, BYK Gardner, Germany). Through surface roughness analysis (Surface Roughness, Surfcorder ET3000, Kosaka Laboratory Ltd, Japan), roughness of the glaze surfaces was analyzed. By using X-Ray diffraction method (Dmax-2500, Rigaku, Japan), an analysis was conducted for crystalline phases in the glaze, and microstructures of glaze surfaces were observed by using a Scanning Electron Microscope (JSM-6707M, Jeol, Japan). For measurement of mechanical characteristics of the glaze, Vickers hardness was measured (Hardness Testing Machine, Mitutoyo, HV-112, Japan). Vickers hardness was analyzed by applying a pressure to the diamond tip according to KS L1603 specification to form an indentation, followed by measurement of the Vickers indentation diagonal length (μm).
3. Results and Discussion
While stable glaze quality free of cracks on the glaze surface was observed in the specimens sintered at 1250°C in oxidizing and reducing atmospheres, the specimens oxidatively sintered at 1315°C showed appearance of cracks on the glaze surface as a whole. The matrix used for experiment was optimized at sintering temperatures of 1220 - 1270°C, and cracks appear to have occurred due to abnormal shrinkage and expansion caused by over-firing as the optimum range was exceeded. In alumina matt regions, devitrification phenomenon commonly occurred, while the texture of silica matt region was observed to be opaque as a rough surface as if insufficient melting had occurred.
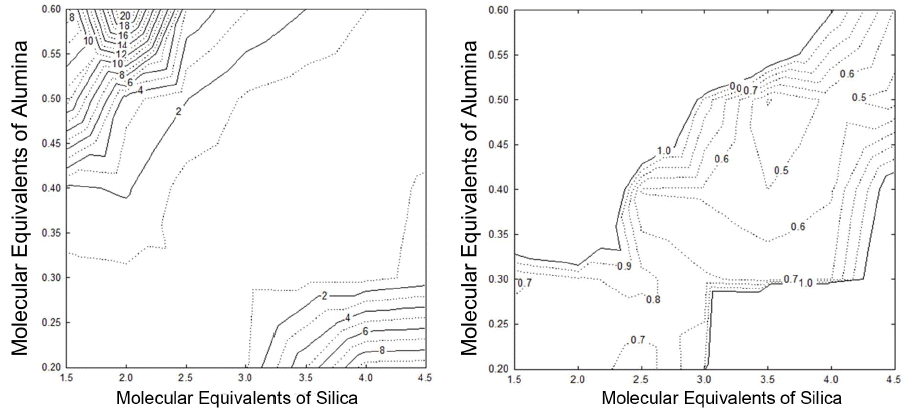
Glossiness values of specimens were analyzed and summarized in Fig. 1. By linkage with the extent of gloss shown in the naked-eye inspection of specimens, the gloss characteristics of glaze was defined as Gloss when the glossiness value at an incident angle of 60° was higher than 70(Glossiness Unit =GU), Semi-gloss when the value was in the range of 70-10(GU)), and Matt when it was lower than 10(GU). Gloss region of the specimens oxidatively sintered at 1250°C was present in the ranges of 0.4-0.5 for Al2O3 content and of 2.5-4.5 for SiO2 content as shown in Fig. 1(a), and the specimen 17 showed the highest glossiness value of 93.4(GU). The semi-gloss region appeared between the gloss region and the matt region, and could be seen to have glossiness distributed over the range of 54.8-35.6(GU), while the matt region appeared in the regions where the contents of alumina and silica were respectively high with the glossiness values being observed in the range of 9.7 - 2.1.
While the gloss region for the specimens reductively sintered at 1250°C appeared in the composition range similar to that for oxidatively sintered specimens, the gloss region can be seen to be generally narrowed down as the semi-gloss region was widened (Fig. 1(b)). The highest glossiness value of 82(GU) was observed in the specimen 27. The semi-gloss region showed the values of 69-21(GU), occupying the widest range. Although the matt region was slightly decreased after reductive sintering, glossiness values of the matt specimens were observed to be lower. Despite the fact that the reductively sintered specimens had the same sintering temperature and rate of temperature rise as with oxidative sintering, the former generally exhibited the same glossiness characteristics as those sintered at lower temperatures.
Gloss region of the specimens oxidatively sintered at 1315°C appeared in the ranges of 0.2 - 0.6 for Al2O3 content and 1.5 - 4.5 for SiO2 content, and the specimen 8 showed the highest glossiness value of 96.9(GU) (Fig. 1(c)). The gloss region after oxidative sintering at 1315°C was observed to be the widest among 3 types of firing conditions. This seems to indicate that the semi-gloss region appearing after oxidative sintering at 1250°C had further progress in formation of glass phase with temperature rise to develop into the gloss region. It is interesting to note that both alumina and silica showed a higher glossiness value in the low composition range than in the high side. Although the semigloss region was relatively decreased, the matt region existed in the range similar to that for oxidative sintering at 1250°C.
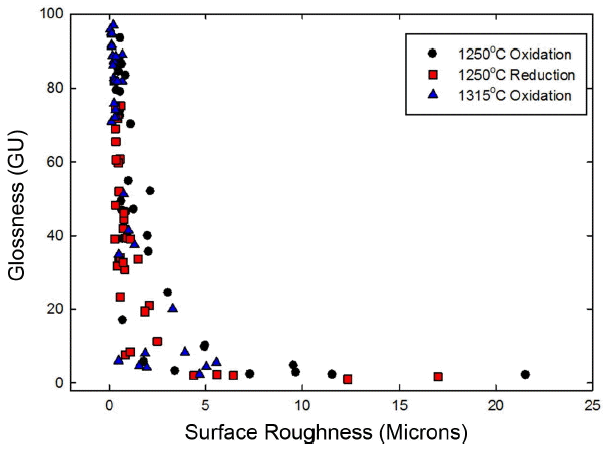
Surface roughness of the oxidative specimens at 1250°C was analyzed and summarized in Fig. 2. Surface roughness of the gloss region under 3 types of sintering conditions was less than 1 μm in average, while that of the semi-gloss region appeared in the range of 0.33 - 4.99 μm, and that of the matt region in the very wide range of 0.49 - 21.54 μm, A correlation between the glossiness and the surface roughness is shown in Fig. 3. As the glossiness value is drastically reduced with an increase in the surface roughness value, the glossiness value was shown to be drastically lowered in the surface roughness range of 1 - 20 μm. Beyond the surface roughness of 20 μm, the glossiness value was lowered to less than 10GU showing no further large change.
Surface roughness values for each gloss region can be seen not to be separated but to appear overlapped. Sintering temperatures had a direct effect on the surface roughness by promoting melting of raw materials, and sintering atmosphere was also observed to have had an effect. Interestingly, surface roughness for the specimens reductively sintered at 1250°C was also lower, while glossiness values of reductively sintered specimens were generally lower as compared with the oxidatively sintered specimens. This shows that there are factors affecting the glossiness in addition to the surface roughness, and the glossiness is presumably affected also by the glass composition of the glaze or the shape of crystals formed on the surface. In alumina matt region, surface roughness showed high values, whereas relatively low surface roughness values were observed in silica matt region.
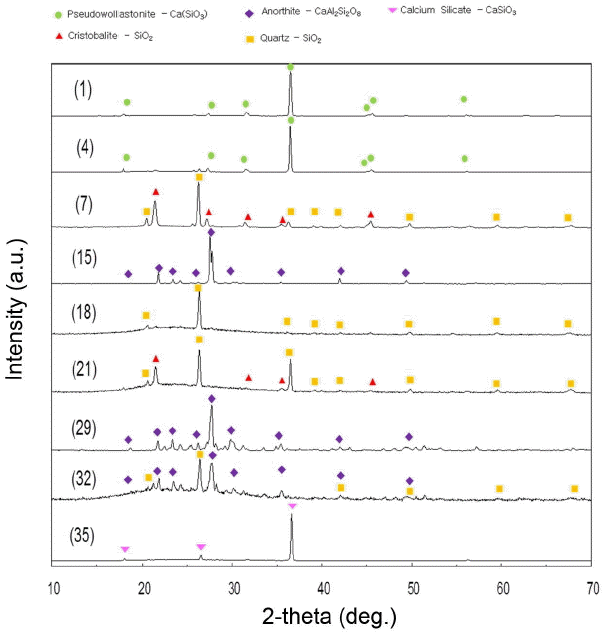
In all of the glaze specimens oxidatively sintered at 1250°C, stable glass without cracks on the surface was formed. Nine specimens belonging to gloss (specimens 18, 21, 35), semi-gloss (specimens 1, 4, 32), and matt (specimens 7, 15, 29) regions were selected for the analyses of crystalline phases and microstructures. As shown in Fig. 4 and Table 1, presence of Quartz, Cristobalite, and Calcium silicate phases was affirmed in the gloss region. In the semigloss region, Quartz, Pseudo-wollastonite, and Anorthite phases were affirmed to be present, while Quartz, Cristobalite, and Anorthite phases were affirmed in the matt region.
Crystalline phases formed per region appear to be directly affected by compositions. When the KNaO composition among glaze compositions used for experiment is assumed as CaO to be represented in the ternary phase diagram of CaO-SiO2-Al2O3, the glaze composition is placed in the region surrounded by Cristobalite, Pseudo-wollastonite, and Anorthite. Thus, the specimens having undergone the same sintering process are expected to form crystalline phases during cooling process according to each composition region.3-4) In the matt and semi-gloss regions with a high alumina content, Anorthite (CaAl2SiO2O8) phase was commonly present, while Quartz phase was commonly present in the gloss and the semi-gloss regions with a high silica content. Also, in the region enriched with calcium and silica compositions, formation of Calcium silicate phase could be affirmed. In the gloss region, it appears that only Quartz phase is left as Calcium silicate or Anorthite phase presumed to be an intermediate compound disappears.
Observations were made on microstructures on the glaze surface and summarized in Fig. 5. The specimens 18, 21, and 35 belonging to the gloss region could be affirmed to have a relatively smooth surface. In the specimens belonging to the gloss region, unmelted silica particles had a form of being embedded in matrix. In the semi-gloss specimens 1, 4, and 32, diversified forms of crystalline phases could be observed. In the specimens 1 and 4 with a low alumina content, a fan-shaped crystalline phase presumed to be pseudo-wollastonite could be observed on the glaze surface, while acicular Anorthite crystalline phase could be observed in the specimen 32 with a high alumina content. In the specimens 7, 15, and 29 belonging to the matt region, characteristic microstructures could be seen depending on the compositional areas. In the case of No. 7 as a silica matt specimen, exposure of unmelted silica on the surface could be affirmed. In the specimens 15 and 29 belonging to alumina matt region, it could be seen that Anorthite crystalline phase was grown to form a rough surface state composed of many pores and a structure of 3D form.5-6)
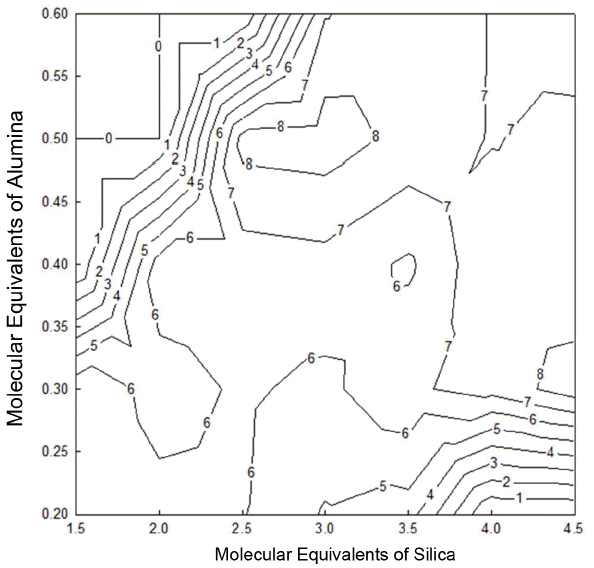
Hardness of the glaze as a function of glaze composition was analyzed and summarized in Fig. 6. In general, high hardness values were observed in the gloss region, and the specimens 24, 25 in the ranges of 0.47 ~ 0.53 for Al2O3 and 2.5 ~ 3.3 for SiO2 as well as the specimen 14 with a high silica content showed the highest hardness value of 8 Hv/GPa. For the specimens 15, 22, 23, 29, 30, and 31 of alumina matt region and the specimens 6 and 7 of silica matt region, indentation marks were not formed due to rough surfaces of the glaze, disabling measurement of hardness. In general, hardness was also shown to be increased with an increase in alumina and silica contents in the gloss region. In the case of glass, the composition having a high glass transformation temperature is also known to have a high elastic modulus and a high hardness.7-8) Hence, hardness value appears to be increased with an increase in alumina and silica contents. However, in the case of glaze, accurate prediction and measurement of hardness is accompanied by difficulties due to non-uniform state where glass phases and crystalline phases are mixed.
4. Conclusions
By fixing the ratio of alkali and alkali earth oxides as a flux composition to be 0.3 : 0.7 with application of UMF, and by varying the ratios of alumina and silica in the ranges of 0.2 - 0.6 and 1.5-4.5, respectively, 35 types of glaze were combined. After sintering the glaze with variation in sintering temperatures and atmospheres, glossiness, surface roughness, crystalline phase, microstructure and hardness were analyzed.
Under 3 types of sintering conditions (oxidation at 1250°C, reduction at 1250°C, oxidation at 1315°C), the glaze was divided into gloss, semi-gloss, and matt regions according to composition areas. The specimens oxidatively sintered at 1250°C had formation of stable glass in the gloss region, while the gloss region was distributed most narrowly after reductive sintering. After oxidative sintering at 1315°C, the gloss region was most widely distributed, and cracking phenomenon was visible on the glaze surfaces. Semi-gloss region appeared at a boundary face between the gloss and the matt regions, while the matt region was observed in the area where the ratios of alumina and silica were relatively high. Devitrification phenomenon appeared in the alumina matt region, while the silica matt region was observed to be opaque having a rough surface as if the glaze composition were not melted.
Glossiness and surface roughness of the glazes exhibited a non-linear, inversely proportionate relationship. Surface roughness of the gloss region was less than 1 μm in average, while that of the semi-gloss region appeared in a range of 0.33 - 4.99 μm, and that of the matt region in a very wide range of 0.49 - 21.54 μm. The glossiness values were drastically lowered in the surface roughness range of 1 - 20 μm, and lowered to less than 10GU beyond 20 μm without showing any further large change.
According to the analysis results of crystalline phases and microstructures, presence of Quartz and Calcium silicate phases was affirmed in the gloss region, that of Quartz, Calcium silicate, and Anorthite phases in the semi-gloss region, and that of Anorthite, Quartz, and Cristobalite phases in the matt region. The gloss region was generally composed of smooth matrix and some crystals, while the anorthite crystalline phase of small needle form precipitated onto the glaze surface during cooling process was observed. In the case of silica matt region, unmelted silica was exposed on the surface, and Anorthite crystals belonging to alumina matte region were affirmed to have grown and to consist of many pores and a structure of 3D form.
Hardness value of the glaze was shown to be the highest at 8(Hv/GPa) in the ranges of 0.47 ~ 0.53 for Al2O3, and 2.5 ~ 3.3 for SiO2 as well as in the specimen 14 with a high Silica content. Hardness values of the glaze were mostly 6 ~ 7(Hv/Gpa) and distributed throughout a wide region.