Lee, Jeon, Song, and Kim: Effect of High-Molecular Weight Organic Compounds on Improvement of Pore Structure of Cement Materials
Abstract
Carbon dioxide emissions involved in global warming are one of the most important issues in the world, and carbon dioxide emissions from the cement industry are about 7% of total carbon dioxide emissions. Thus, reduction in the amount of utilized cement can contribute to a reduction of carbon dioxide emissions. The average life of concrete is 20 ~ 30 years, and if concrete life can be improved by ten years, cement use will be much lower. In this study, we examined the use and effect of fructan from microbes as a method for the densification of the pore structure of cement. The effect of fructan on the hydration reaction and pore distribution, as well as the water absorption of hardened cement mortar were studied. Pores distribution increased in mesopore OPC, and absorption rate was found to decrease with the use of fructan, which has a glue-like and swelling character.
Key words: Fructan, Microbe, Levan, Absorption, Pore distribution
1. Introduction
Concrete experiences deterioration due to various factors such as materials, mixture ratio, manufacturing method, curing conditions, and environment. Deterioration usually involves water, and the extent of deterioration in porous materials is determined by permeability. Ordinary Portland cement (OPC), used as a binder, is a porous material with pores in the range of 10 μm to 0.5 nm after hardening. The pores of hardened OPC can be classified into two groups: gel pores in calcium silicate hydrates (CSH) and capillary pores produced from evaporation of water. 1) These pores allow COx, SOx, Cl ions, and H 2O to infiltrate the hardened OPC, and the crystallization pressure of the resulting salts results in cracks and freezing and thawing, thus reducing the durability of concrete. 2)
To enhance concrete durability, research has been conducted on self-healing of cracks. The self-healing of concrete cracks can be achieved through the use of various organic and inorganic materials. One new attempt in this area is the use of microorganisms. While the use of microorganisms for self-healing of concrete cracks is highly valuable in theory, some doubts have been raised regarding its practicality, especially considering the difficulty of raising microorganisms that match the conditions of crack formation in concrete. 3)
This study applies an intermediate product of bacteria to cement materials and assesses the possibility of enhancing durability by introducing a change in the porous structure of a cement hardener, with the aim of overcoming the difficulty of controlling the growth conditions of microorganisms. The microstructure of the cement hardener was examined using microbial fructan (levan), which acts as levan glue similar to the intermediate product BacillarFilla that is being used by researchers at Newcastle University 4) to examine the characteristics of concrete crack healing.
2. Experimental Procedure
Fructan, the high-molecular organic compound used in this study, is a polymer of fructose molecules. It is a single polymer that connects up to millions of fructose, and has many different types. Type 2 is known to be highly reactive with metal ions (Fe, Ca, Zn, etc.), and does not cause environmental pollution. Depending on the type of microorganism produced, fructan exhibits different physical properties due to changes in molecular weight, degree of polymerization, and degree of branching. 5)
The microbial fructan levan (‘FL’) employed in this study was a product of Realbiotech Co. Ltd., and its molecular structure and FT-IR results are presented in Figs. 1 and 2. Peaks are observed at 3300 cm −1 for O-H, at 2900-2800 cm −1 for C-H, at 1600 cm −1 for C=O, at 1200-900 cm −1 for polysaccharide functional groups, and lastly at 900 cm −1 and 800 cm −1 for furanoid rings of the levan monomer. 6)
A characteristic of FL is that glucose is connected to fructose in the form of β-2, 6. 7) The average molecular weight of FL was measured using Tosoh’s EcoSEC HLC-8320 GPC and was found to be approximately 540,000. The cement used in the experiment was ordinary Portland cement Type 1 (Sampyo Tongyang, Co., Korea), having the chemical composition and physical properties shown in Tables 1 and 2. In the experiment, cement was substituted by FL at 0.1-0.3 wt.% to examine the effects on the hydration reaction and porous structure of cement. The experimental plan is provided in Table 3. The cement paste had a W/C of 0.4, and the experiment proceeded with hydration heat measurement, a hydration product analysis, and a porous structure analysis. The mortar was prepared as rectangular specimens measuring 40 × 40 × 160 mm in accordance with the standard method of cement strength testing (KS L ISO 679) and cured underwater. Compressive strength was measured after 3, 7, and 28 days of aging. Hydration heat was measured in an insulated state to prevent influence from outside temperatures using a K-type thermocouple and a 10-point data logger (UCAM-60B, Kyowa Co., Japan) at 1-second intervals. To analyse the hydration products, hydration was stopped with acetone at ages of 3, 7, and 28 days. The specimens were then dried in a dryer at 40°C for 24 h, and ground into sizes below 200 μm for XRD (Rigaku Co., D/Max-2500V) analysis with a 2θ of 5-80° at 4°/min. The porous structure was analysed with the same hydration stopping as XRD, and mercury intrusion porosimetry (MIP) was employed while keeping the specimens at a consistent size of 4-5 mm. Auto Pore IV 9520 of Micromeritics was used in the MIP, and the maximum pressure was 60,000 psi. The range of pore diameter for the measurements was 360 μm-3 nm. The absorption rate was measured up to 72 h using the method of measuring the water absorption coefficient for construction materials (KS F 2609).
3. Results and Discussion
The results of hydration temperature measurements are shown in Fig. 3 and Table 4. For specimens containing FL, the hydration temperature increased as in the control group after 3 h and exhibited characteristics of hydration heat similar to the control. As shown in the setting time measurements in Fig. 3, the final setting time was delayed by the addition of FL, while the initial setting time was similar to that of the control. The addition of FL tends to cause a delay in the setting time. In general, carboxyl radicals (OH −) and carboxyl groups are adsorbed on the surface of cement particles, and delay or interfere with the initial hydration of cement. FL does not significantly influence the hydration temperature at the initial stage of hydration because the molecular structure of fructose is more closely related to the dissociation rate of carboxyl radicals (OH −) and carboxyl groups compared to other saccharides. The delay in setting time is due to the formation of complex salts by fructan, with a molecular weight higher than 540,000, and Ca 2+ ions, which contributes to suppressing the precipitation of Ca(OH) 2 crystals and interferes with hydration. 8) In the hydration of cement materials, the formation of complex salts by Ca 2+ ions delays the setting time, and is known to have the same effect on both the initial and final setting times. However, this study found that the difference between initial setting time and final setting time arises from the difference in dissociation of carboxyl radicals (OH −) and carboxyl groups in high molecular weight FL. For high molecular weight FL, the amount of complex salts is minimal during the gradual dissociation of carboxyl radicals (OH −) and carboxyl groups, but increases rapidly when dissociation is complete. For high molecular weight fructose, further study is needed to examine whether there are differences in the rate of dissociation of carboxyl radicals (OH −) and carboxyl groups under alkaline conditions. As shown in Fig. 4, the analysis of hydration products revealed that all products had characteristics similar to the control at ages of 3 and 28 days. The primary products were portlandite and ettringite. Since there was no difference in the peak intensity of each product, we can presume that fructose does not have a significant influence on hydration reactions of cement pate in the initial stage. In general, saccharides cause a delay in hydration reactions when mixed with cement, and are characterized by a low hydration temperature and a high degree of hydration. However, in the case of levan comprising fructose polymers, there was neither an initial delay nor influence on the initial cement hydration. This is consistent with the findings on hydration heat characteristics in the initial stage. According to Metha and Monterio, the pore size of cement hardeners can be classified into gel pores (< 4-4.5 nm), mesopores (4.5-50 nm), medium capillary pores (50-100 nm), and large capillary pores (>100 nm). 9) Fig. 5 gives the results of MIP, conducted to examine the effects of levan on the pores of the cement hardener. The total porosity can be found in Table 5. The pore distribution results show a decrease in the porosity of the FL-based hardener at the ages of 3 and 28 days compared to the control. In particular, at 3 days of aging, there was a rapid decrease in pores larger than 0.1 μm. At 28 days of aging, pores smaller than 0.01 μm grew smaller compared to that of the control. The capillary pores of cement hardener are known to be significantly influenced by factors such as diffusion, absorption, and permeability. To examine these effects, the pores were classified by size, as shown in Fig. 6. With the addition of FL, the amount of capillary pores decreased at 3 days, but were present in a greater amount compared to the control at 28 days. Because the total porosity remained the same, the addition of FL is presumed to have caused an increase in the amount of gel pores. In the presence of FL, the hydration products increased more rapidly compared to the control. The results of compressive strength measurement are shown in Fig. 7. The compressive strength is weaker than that of the control at ages of 3 and 7 days, but becomes similar or stronger at 28 days. Similar to pore distribution, these results can be traced to the more elaborate hydration products and the filling of gaps with the aggregate by the FL glue. The changes in absorption rate were measured according to the procedures of KS F 2609. 10) The S/B of mortar was varied to 2 and 3, and measurements were obtained after 1, 6, 24, and 72 h. As shown in Fig. 8, the change in absorption rate over time decreased with an increasing amount of levan for both S/B values of 2 and 3.
Figure 9 compares the porosity of pores larger than 50°C (meso pore & capillary pore) at 28 days and the absorption rate after 72 h of immersion. The graph shows that the absorption rate decreases with an increase in the amount of capillary pores. 11) The porosity is similar for pores of at least the size of mesopores, but the absorption rate decreased with the addition of FL. This can be traced to the characteristics of FL glue and swelling. FL exhibits the characteristics of glue and experiences swelling when in contact with water, which reduces the pores through the binding of gaps between hydrated or unhydrated cement particles, and leads to moisture resistance at the same time.
A chloride penetration test was performed to examine the durability of cement mortar in relation to FL addition. Chloride ions penetrate and diffuse through concrete and cement hardeners by penetrating the pores and spreading through free water. 12) The test was conducted according to the standard method of measuring the chloride ion penetration of concrete (KS F 2737), 13) and the changes in the penetration depth of the mortar were examined using an indicator. The specimens were cured underwater for 28 days, and the penetration test was conducted using 5% NaCl solution at 3, 14, 28, and 56 days. The results are shown in Fig. 10. With the introduction of FL, the cement hardener showed excellent resistance to penetration of chloride ions. Similar to the results for absorption rate, the amount of capillary pores increased slightly with the use of FL, and outstanding chloride resistance was observed. The FL glue reduces the pores through binding of gaps between hydrated or unhydrated cement particles, while chloride resistance is enhanced as swelling has the effect of filling the gaps. The decrease in total porosity indicates a denser structure that makes it difficult for salts to spread.
4. Conclusion
When using levan, a type of microbial fructan, the following observations were made:
The initial setting time of the cement hardener was not significantly influenced, but the final setting time was slightly delayed. This can be traced to the rate of dissociation of carboxyl radicals (OH−) and carboxyl groups in high molecular weight FL. The compressive strength of the cement hardener was weaker than that of the control at ages of 3 and 7 days, but became similar to the control at 28 days. The cement hardener showed a decrease in absorption rate due to the characteristics of FL glue and swelling. The cement hardener showed excellent resistance to penetration of chloride ions. The FL glue reduces the pores through binding of gaps between hydrated or unhydrated cement particles, and the chloride resistance is also enhanced with gaps being filled by swelling.
Acknowledgments
“This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education (2017R1D1A1 B03034020)”.
Fig. 1
Molecular structure of levan. 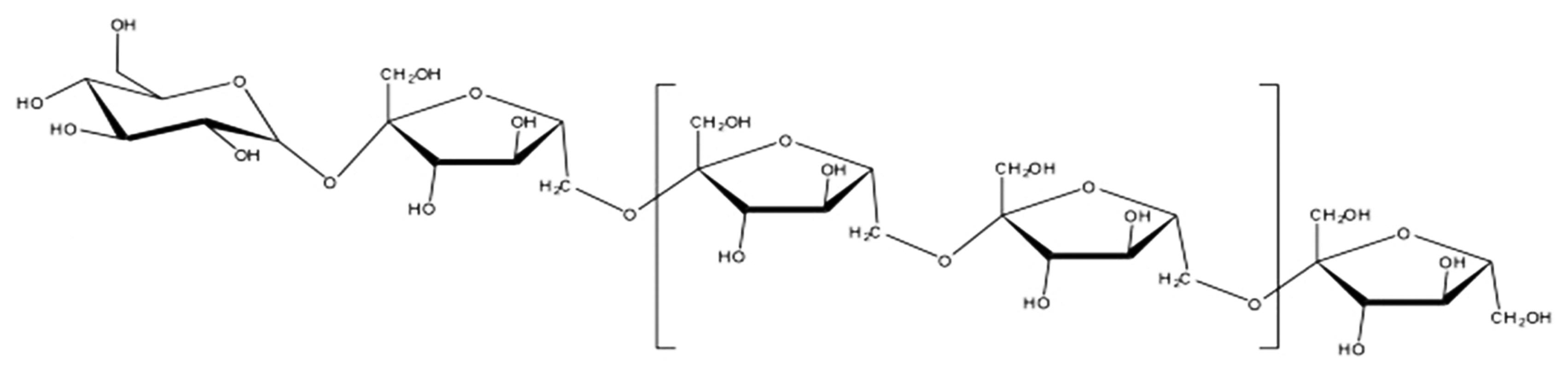
Fig. 2
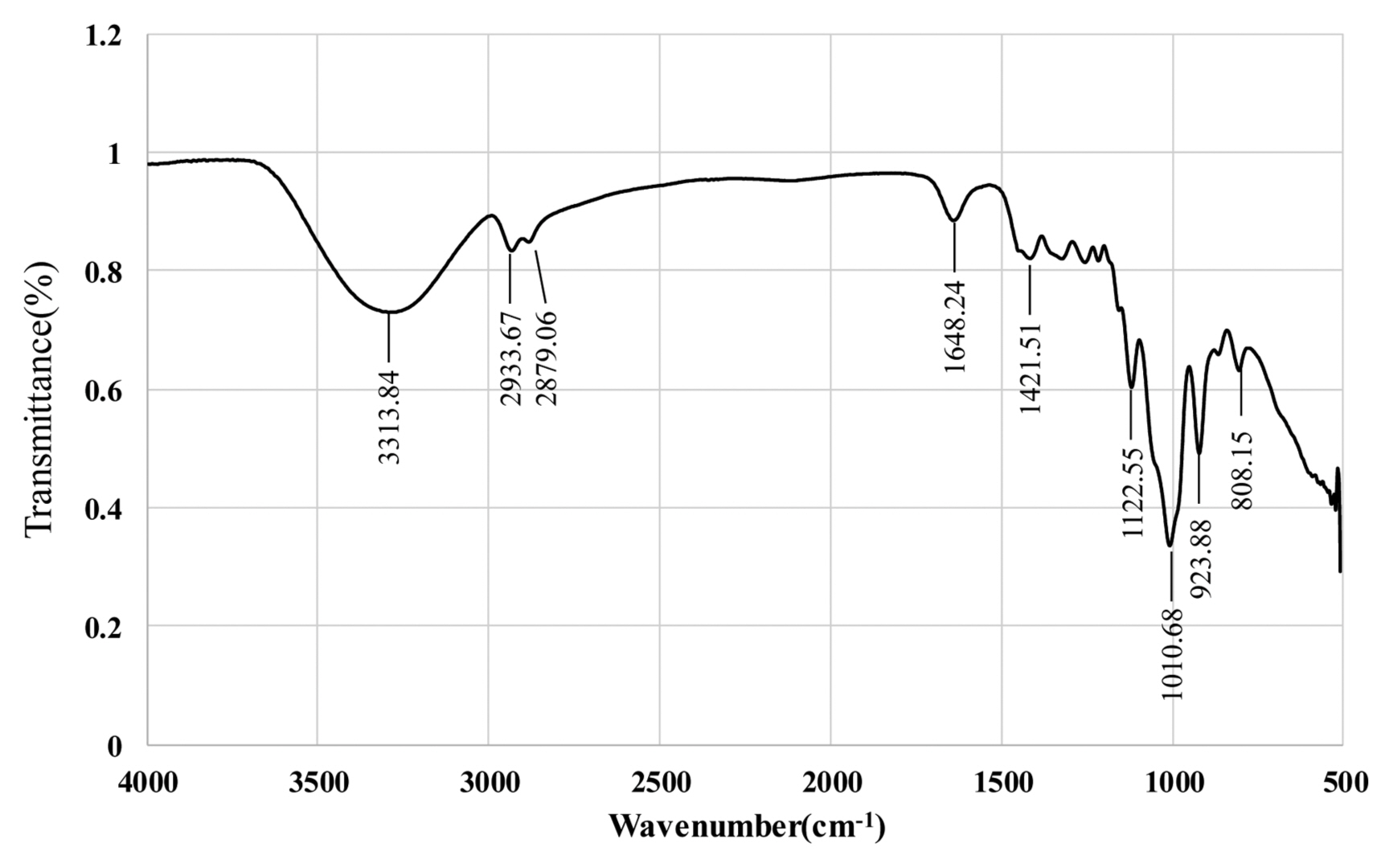
Fig. 3
Hydration temperature and setting time. 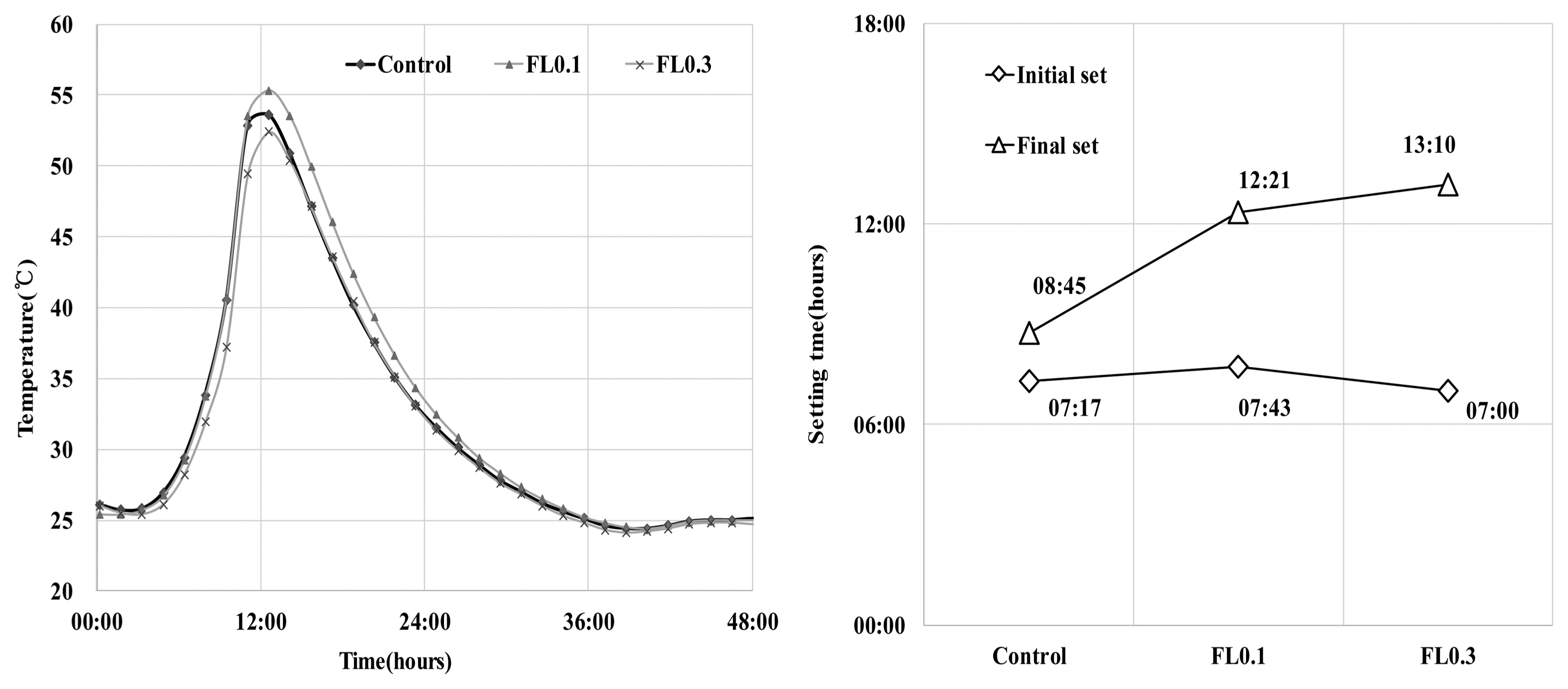
Fig. 4
Results of X-ray diffraction. 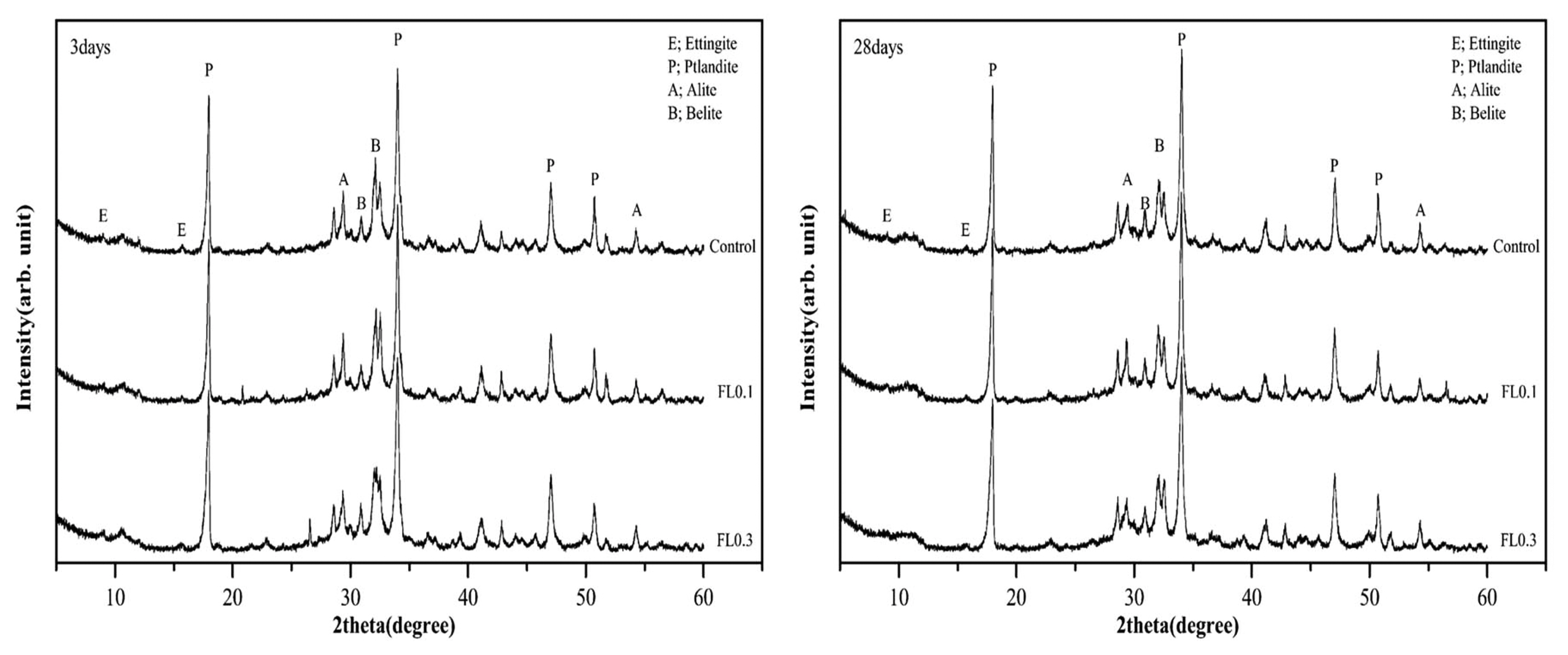
Fig. 5
Pore volume distribution. 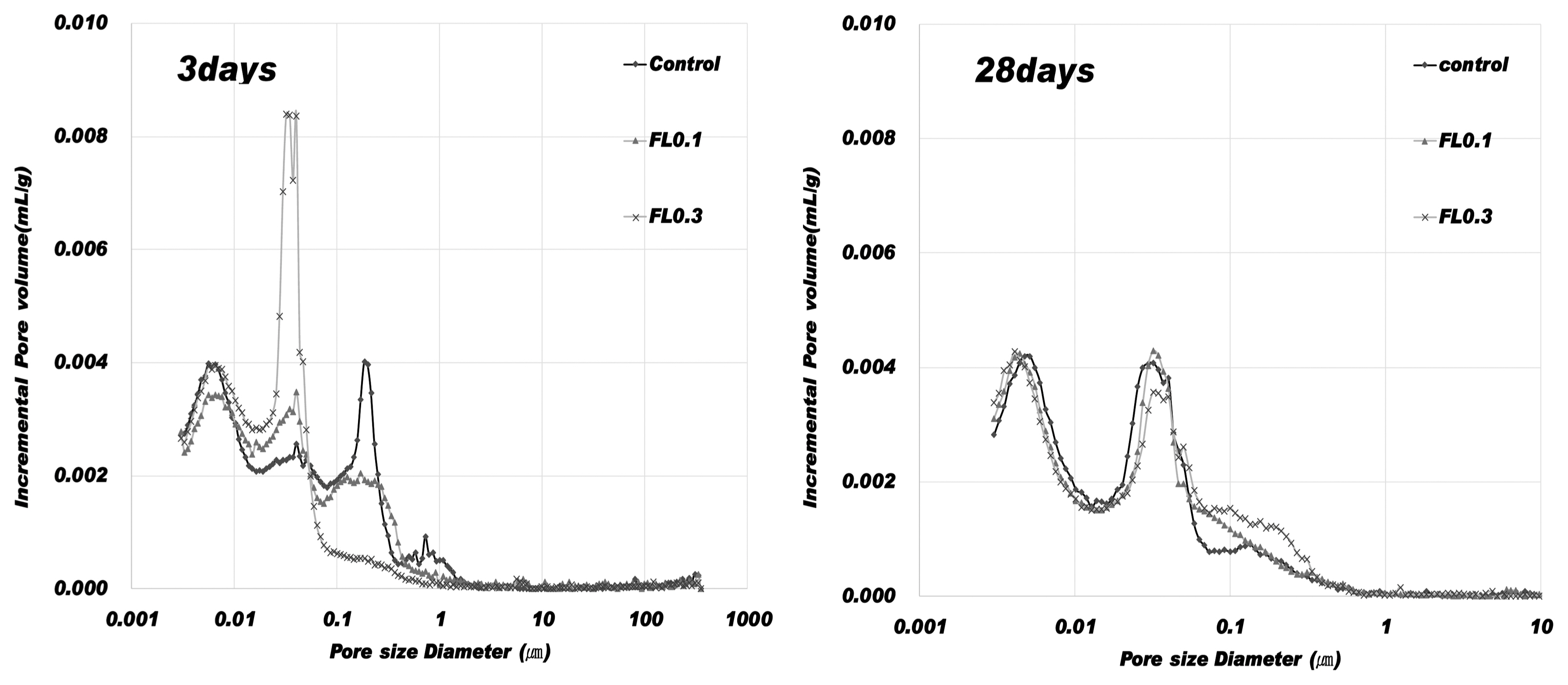
Fig. 6
Comparison of pore size distribution. 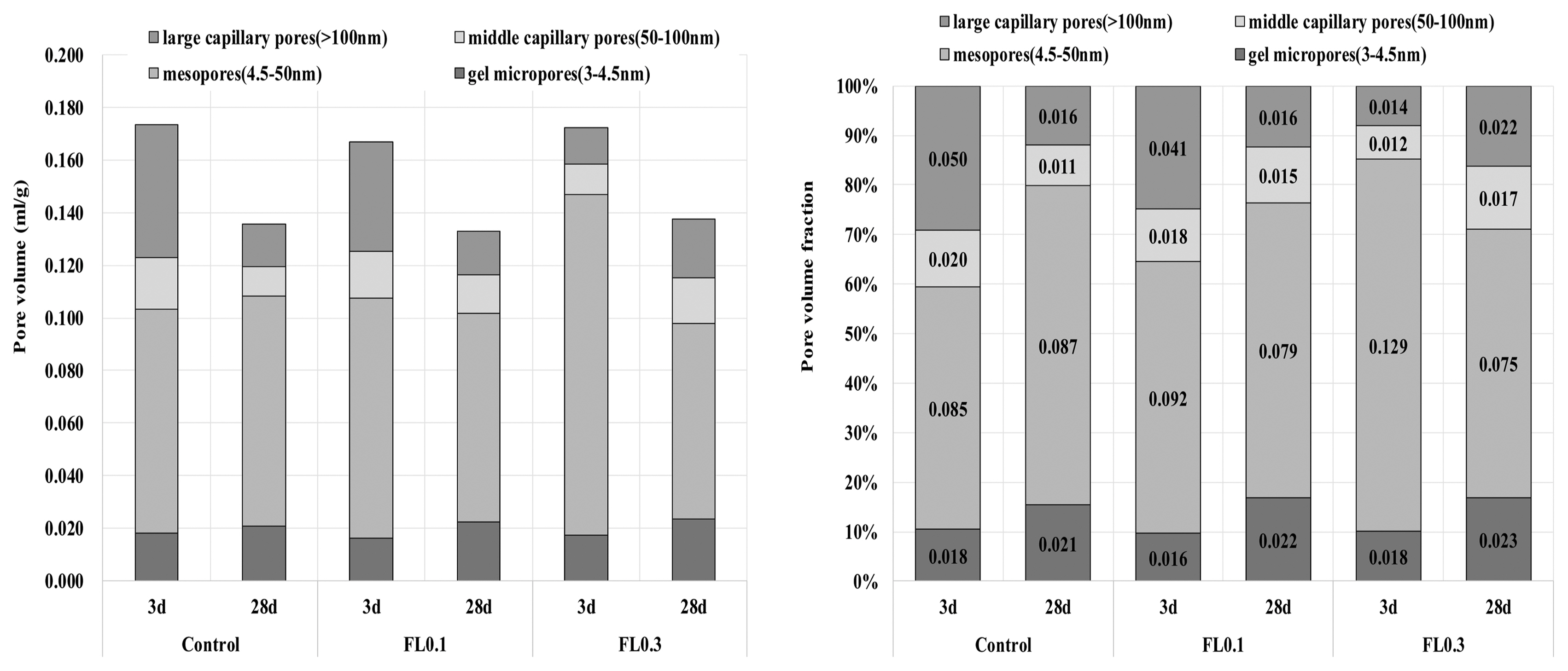
Fig. 7
Results of compressive strength 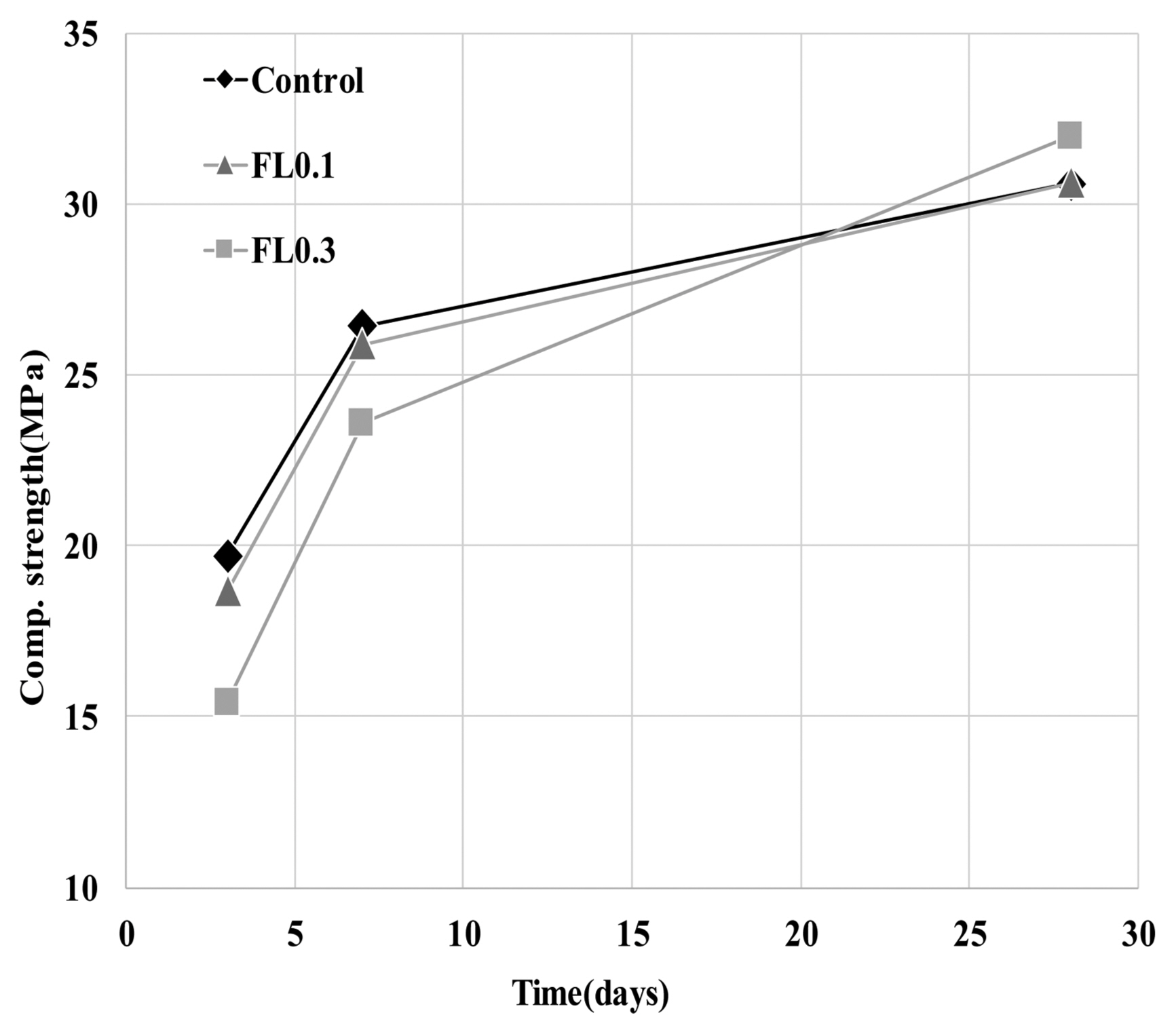
Fig. 8
Change in water absorption rate with time. 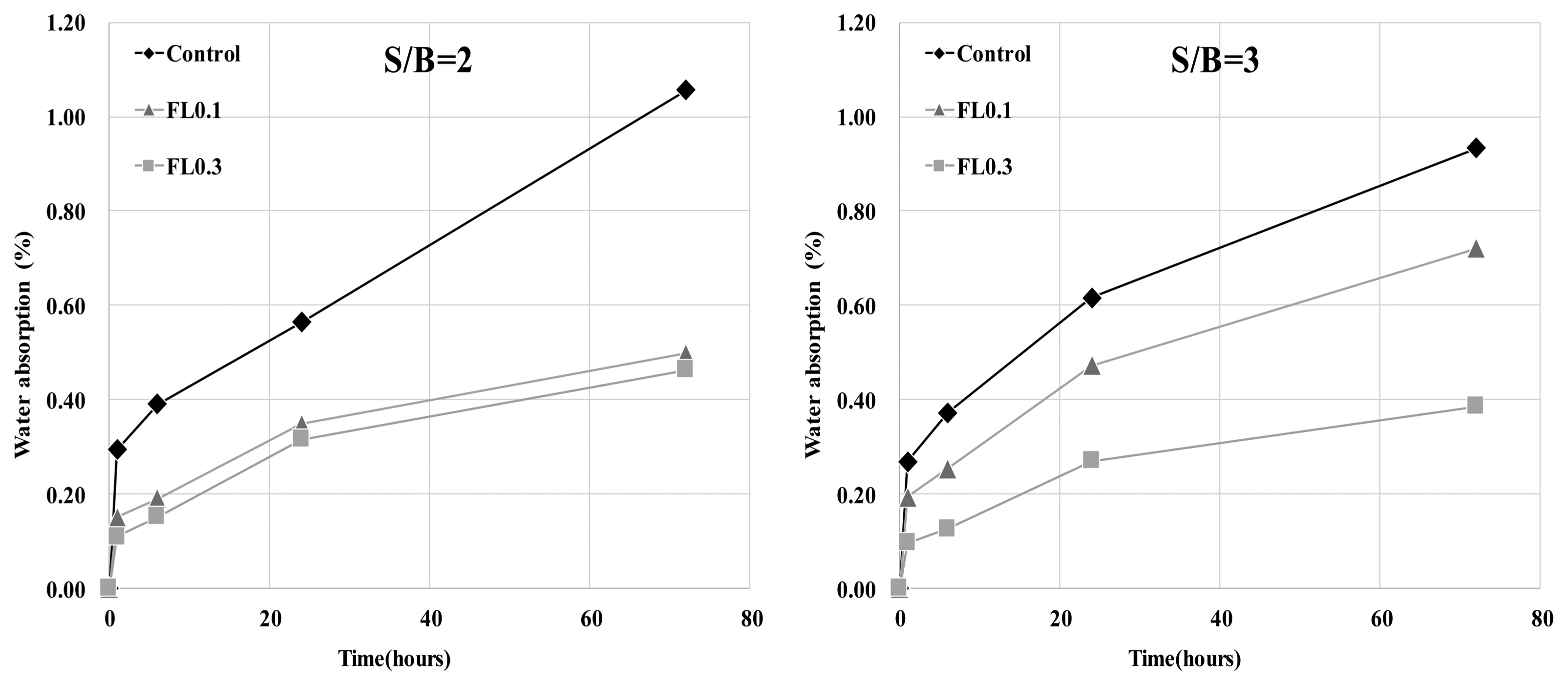
Fig. 9
Relationship between capillary pores and mesopores by absorption. 
Fig. 10
Results of chloride ion penetration depth. 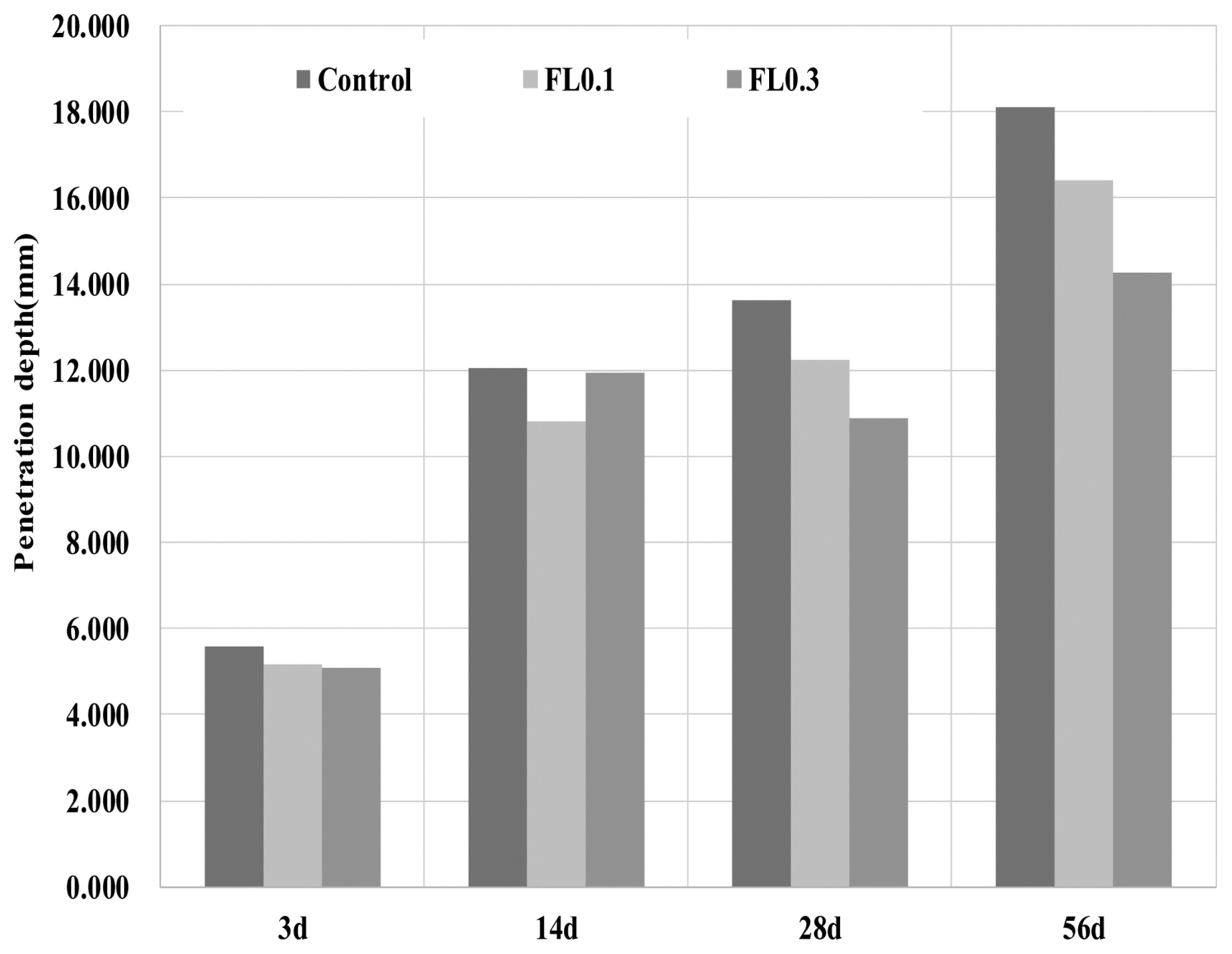
Table 1
Chemical Composition of OPC
|
Component |
SiO2
|
Al2O3
|
Fe2O3
|
CaO |
MgO |
K2O |
TiO2
|
Mn2O3
|
P2O5
|
SO3
|
LOI |
|
Content (%) |
20.6 |
5.39 |
2.91 |
61.50 |
3.67 |
1.04 |
0.34 |
0.15 |
0.11 |
2.17 |
1.04 |
Table 2
Physical Properties of OPC
|
Specific gravity |
Blain (cm2/g) |
Setting time (min) |
Compressive strength (kg/cm2) |
|
3.15 |
3,302 |
Initial |
Final |
3 day |
7 day |
28 day |
|
237 |
341 |
245 |
301 |
387 |
Table 3
|
Conditions |
Remarks |
|
Mix |
Replacement |
3 |
0, 0.1, 0.3 wt.% |
|
W/B |
1 |
0.5 |
|
S/B |
2 |
2, 3 |
|
|
Measurement |
Com. strength |
3 |
3, 7, 28 days |
|
Hydrating temp. |
1 |
- |
|
Absorption |
2 |
72 h |
|
|
Analysis |
Porosimeter |
- |
7, 28 days |
|
XRD |
- |
Table 4
High Temperature and Time to Reach High Temperature
|
Control |
FL0.1 |
FL0.3 |
|
Time (h) |
12:35 |
12:35 |
12:35 |
|
High temp. (°C) |
53.6 |
55.3 |
52.4 |
Table 5
|
Contorl |
FL0.1 |
FL0.3 |
|
3 days |
29.33 |
29.17 |
28.89 |
|
7 days |
28.85 |
27.10 |
27.30 |
|
28 days |
24.12 |
23.65 |
24.16 |
REFERENCES
1. PK. Metha, and PJM. Monterio, Concrete, Microstructure, Properties and Materials; pp. 133-205 McGraw-Hill, London, 2006.
2. M. Duncan, CM. Richard, YH. William, and TG. Kenneth, “Cement-Aggregate Reaction in Concrete,” J Am Concr Inst, 19 93-128 (1947).
3. K. Bae, “A Study on Relationship Between Osmosis by Semi-permeability to NaCl solution and Pore Structure of Cement Mortar,” J Archit Inst, 22 [1] 103-10 (2006).
5. R. Siddique, and NK. Chahal, “Effect of Ureolytic Bacteria on Concrete Properties,” Constr Build Mater, 25 [10] 3791-801 (2011).  6. KH. Jang, and SA. Kang, “Research and Market Trends in Levan,” Food Sci Ind, 36 [2] 85-91 (2003).
7. GO. Phillips, and PA. Williams, Handbook of Hydrocolloide; pp. 604, Woodhead, Cambrige, 2009.
8. JF. Young, “A Review of Mechanisms of Set-Retardation in Portland Cement Pastes Contacting Organic Admixture,” Cem Concr Res, 2 [4] 415-33 (1972).  9. Q. Zeng, K. Li, T. Fen-Chong, and P. Dangla, “Pore Structure Characterization of Cement Pastes Blended with High Volume Fly-Ash,” Cem Concr Res, 42 [1] 194-204 (2012).  10. Korea Standard, Determination of the Water Absorption Coefficient of Building Materials; 2008.
11. PK. Metha, and PJM. Monterio, Concrete, Microstructure, Properties and Materials; pp. 138-42 McGraw-Hill, London, 2006.
12. HS. So, JS. Oh, and GB. Park, “Permeability and Transport Mechanism of Media into Concrete,” Infrastruct Saf, 37 [1] 53-77 (2011).
13. Korea Standard, Testing Method for Chloride Ion Penetration of Concrete by Using Indicator; 2005.
|
|






















