1. Introduction
Fe-based materials are typical inorganic pigment materials; they are non-toxic and photo-chemically inactive, and are required for pigment applications.1-9) The materials exhibit various colors depending on the composition of the oxidized crystallization. Particularly, iron oxyhydroxide (FeOOH) exhibits different hues depending on the crystal structure of α-FeOOH (yellowness), β-FeOOH (yellowish red), and γ-FeOOH (reddish brown). The synthesis of FeOOH pigments is highly influenced by the presence of salt ions such as the effect of various anions (e.g., NO3−, SO42−, F−, Cl−, and Br−) on the microstructure, morphology, and size of the nanoparticles. In comparison, Fe(NO3)3 and FeCl3 salts favor the formation of goethite (α-FeOOH) and akaganeite (β-FeOOH), respectively, and the Fe2(SO4)3 salt leads to the formation of an amorphous mixture of goethite and schwertmannite.4) Goethite is mainly synthesized under basic conditions and akaganeite is synthesized under acidic conditions. In previous studies, we used FeCl3 salt to control the length and coloration of β-FeOOH rods in an acidic environment.5) Goethite, which exhibits a vivid yellow color, is a rod-shaped, one-dimensional tunnel structure derived from mutual bonding between Fe ions and OH.3,10) However, it is difficult to control the shape and coloration of the nanorods. In synthesis, the formation of nanorods is largely dependent on environmental factors such as the pH and reaction temperature due to the bound OH, which easily induces a phase transition to α-Fe2O3.11-14) Therefore, the effects of various reaction conditions have been studied for the synthesis of the α-FeOOH phase.15-17)
In this study, α-FeOOH rods were synthesized using a strong alkali (KOH) as a reaction catalyst. The crystal structure and morphology of the rods, according to the content of KOH participating in the reaction, were investigated. In addition, the variation in the chromaticity according to the length and width of the α-FeOOH rods was optimized and the aspect ratio in a yellow inorganic pigment was derived.
2. Experimental Procedure
Iron (III) nitrate nonahydrate (Fe(NO3)3·6H2O, 99%, Daejung Chem., Korea) and potassium hydroxide (KOH, 99%, Duksan Chem., Korea) were used without further purification as starting materials for the synthesis of α-FeOOH. First, 0.1 mol of Fe(NO3)3·6H2O as a Fe precursor was dissolved in 100 ml of distilled water. When the Fe precursor was completely dissolved, KOH, which is a reaction catalyst, was added at various concentrations. The reaction solution containing KOH was sonicated until the KOH in the solvent was completely dissolved. Next, the solution was transferred to an oven and heat-treated at 85°C for 12 h. We used a closed system to prevent the evaporation of water. After the reaction was completed, the precipitate was separated from the solution by centrifugation at 6,000 rpm. The obtained product powders were washed several times in distilled water and under the same conditions as those for the previous centrifugation. Subsequently, the powders were dried at 60°C for approximately 24 h to obtain the final powder.
The morphology of the prepared pigments was evaluated by transmission electron microscopy (TEM; TEM-2010, JEOL, 300 kV, Kyoto, Japan). The crystal structure of each sample was analyzed by X-ray diffraction (XRD; Rigaku D/max 2500v/pc, Japan) under CuKα radiation (λ = 1.54178 Å). The CIE Lab color parameters were measured based on the KOH concentration, and the pigments were analyzed by UV-visible spectroscopy (UV2600, Shimadzu, Japan).
3. Results and Discussion
Figure 1 shows the TEM images of the prepared pigments according to the content of KOH as the reaction catalyst. The morphology of the reactants with the lowest KOH content of 0.1 mol was a lump-like compound that did not retain the shape of the particles. These lumped compounds underwent changes in shape to such an extent that they could be regarded as spherical when the KOH content increased to 1 mol. The pigment shape underwent a drastic change with increase in the KOH content to 5 mol. In this case, fine needle-shaped powders were entangled with each other. Furthermore, for 10 mol KOH, the overall size of the synthesized rods increased greatly compared to that of the rod formed using 5 mol KOH. Although the aggregate particles did not dissociate, they finally had a basic rod shape. The increase in the reaction catalyst content gradually promoted rod-based uniaxial growth. As can be seen in Fig. 1(e) to (g), the rods became longer and thinner depending on the KOH content used as the catalyst. However, it was confirmed that when the KOH content exceeded a certain amount (30 mol), uniaxial growth stopped and a secondary shape, such as another type of impurity, was formed.
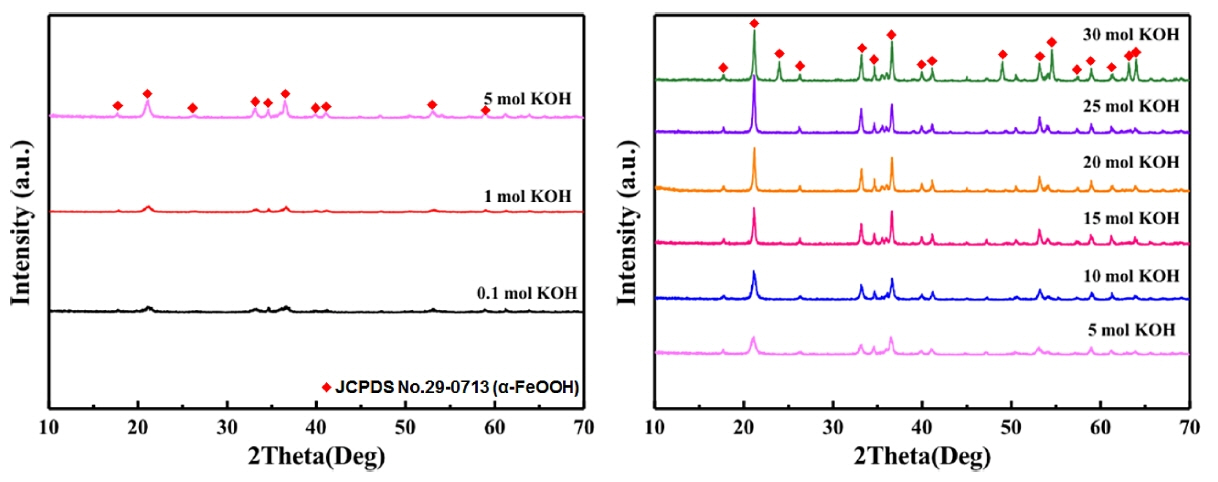
For crystal structure analysis, Fig. 2 provides the XRD patterns according to different KOH contents. For the sample with 0.1 mol KOH, very-low-intensity peaks were formed. It is considered that a compound with such a low-intensity peak will not undergo sufficient crystallization. On correlating these results with those from the microstructure analysis, this low crystallinity can be regarded as the reason for not properly attaining a rod shape. On the other hand, distinct peaks appeared in the sample with 5 mol KOH, in which rods began to form. The 2θ diffraction angles corresponding to the peak positions were 21°, 26°, 33°, 36, and 53°. Hence, the synthesized crystal phase was α-FeOOH with an orthorhombic structure, as per the data in JCPDS No. 29-0713. Therefore, the KOH content of the catalyst has great influence on the crystallization of α-FeOOH. The intensity of the diffraction peak changed according to the increase in the relative amount of KOH. Particularly, there was a gradual increase in the intensity of diffraction at 2θ ≈ 21°, which corresponds to the (110) plane. It can be seen that the increase in the length of the synthesized rod with the increase in KOH content is due to the overall crystallinity structure. That is to say, the OH of KOH as a catalyst promoted the growth of the (100) plane, which is the most crystalline crystal plane; hence, uniaxial growth was thought to have been achieved. On the other hand, the microstructure of the crystal part of the synthesized pigments exhibited not only rod shapes but also secondary forms; 25 mol of KOH produced diffraction peaks at 2θ ≈ 24° and 49°, as well as the conventional diffraction peaks of α-FeOOH. These diffraction peak patterns represent those of α-Fe2O3, which has a rhombohedral structure, and correspond to the (012) and (024) planes, respectively. As can be seen, the secondary phase identified in the microstructure analysis results from a phase transition to α-Fe2O3 due to the excess KOH-induced reaction rate difference. Therefore, it is considered that the content of KOH increases the amount of OH promoting the reaction, thereby controlling the particle size and acting as a factor to induce phase transition.
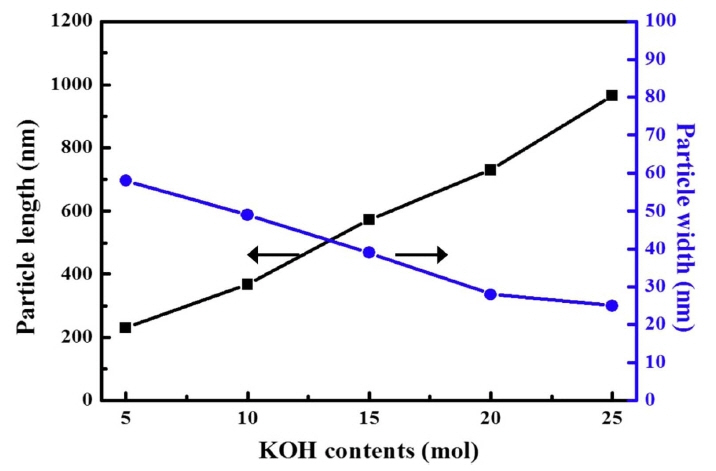
Figure 3 shows the variation in the length and width of the synthesized rods according to KOH content. The pigments synthesized into rod shapes using 5 mol KOH were approximately 512 nm long and 904 nm wide. Initially, the pigments tended to be wider due to the fine needle-shaped aggregation. With subsequent increase in the amount of KOH added, the size of each axis increased continuously. Finally, when 25 mol of KOH was added, the aspect ratio of the rods increased sharply. The length and width were approximately 854 nm and 43 nm, respectively.
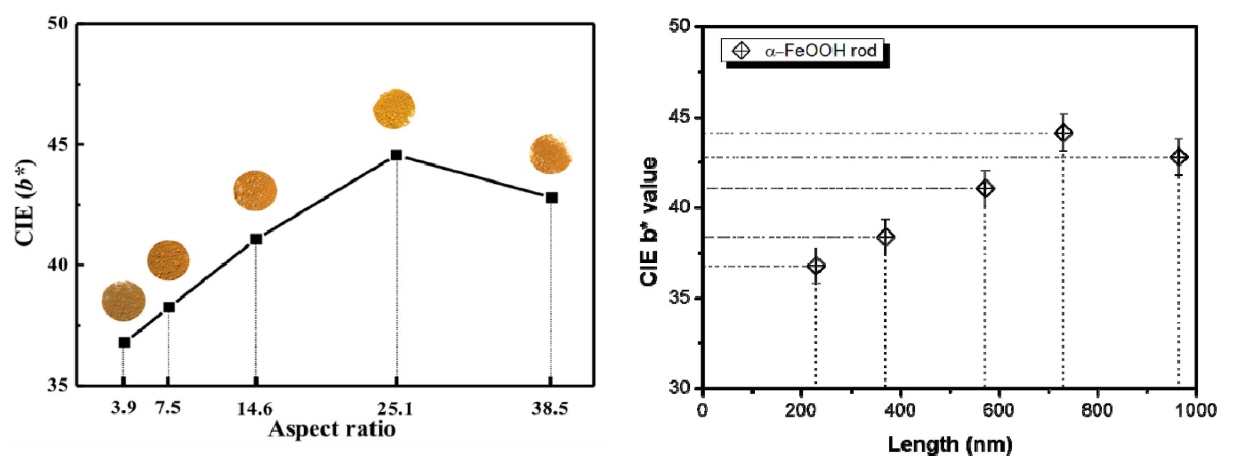
Figure 4 and Table 1 show the chromaticity CIE Lab analysis according to the aspect ratio of the synthesized α-FeOOH. Chromaticity in CIE Lab is an index that uses the values of brightness (L*), red/green (a*), and yellow/blue (b*). When the a* and b* values are positive, it is possible to deduce the degree of redness and yellowness.3,5) α-FeOOH is an inorganic pigment, and is considered to be most important characteristic of yellowness, as mentioned above. Therefore, the CIE b* value indicates initial crystallinity. The sample with the shortest and thickest particle, with an aspect ratio of 3.9, had a b* value of approximately +36.80. The yellowness improved with increasing aspect ratio, such that the CIE b* value increased to +44.14. However, the thinnest α-FeOOH rod, with an aspect ratio of 38.5, had a CIE b* value of +42.80. Hence, the yellowness increases as the aspect ratio of α-FeOOH increases; however, this tendency was reversed beyond a critical point. Therefore, an optimum length and width ratio of the synthesized α-FeOOH rods should be determined to realize distinct yellowness. The CIE a* value, which is a measure of redness and can affect the yellowness, was +25.45 in the initial stage of crystallization; however, it decreased to approximately +15 when the shape of the rod was properly adjusted. The CIE L* value, representing the brightness, was similar overall for the samples, at approximately +56.
Figure 5 plots the UV-vis reflectance according to the aspect ratio of the α-FeOOH rods. In general, the UV-vis reflectance is high in the range of 550 to 750 nm. The color of a material is established through reflection in a specific visible light region; the visible light range corresponding to wavelengths 570 to 780 nm, in particular, provides yellow-red color. The synthesized α-FeOOH rods could be used to identify the reflectance peak in a wavelength range from 585 nm to 650 nm, and reflectance changes due to the difference in length. This reflectivity, like the former yellowness characteristic, changes with the height of the peak and the change in the CIE b* value. In conclusion, the aspect ratio of α-FeOOH rod particles improves the reflectivity as well as the yellowness.
4. Conclusions
α-FeOOH rods were prepared using a closed system between Fe nitrate and KOH. Crystalline structures were synthesized according to KOH content and their shapes were determined according to the relative OH content in the reaction. Increased content of crystallized KOH resulted in an increase in the aspect ratio of the rods and the relative OH content. However, when the critical content was exceeded, the rods did not maintain the phase, and a partial secondary oxide α-Fe2O3 phase formed. The optical properties of the α-FeOOH rods gradually increased with increasing aspect ratio. However, beyond the critical aspect ratio (25.10), there was a decrease in yellowness. Thus, the relative optimal aspect ratio of this material for use as a yellow inorganic pigment was derived.