1. Introduction
Solid oxide fuel cells (SOFC) produce electricity by the reaction of oxygen and hydrogen at elevated temperature producing water as a byproduct and have been extensively studied as a clean alternative energy source.1) On the contrary, solid oxide electrolysis cells (SOEC) decompose water with electricity producing oxygen and hydrogen gases.2) Since a SOFC can be also used as a SOEC and vice versa when the device is operated reversely, a reversible solid oxide cell (RSOC) can be a good alternative power source for small and medium sized buildings and thus also has been widely investigated.3-5) As a RSOC operates under the same conditions as a SOFC, hermetic sealing of RSOC components to prevent any possible leakage of gases is crucial for the efficiency and long-term stability of the cell. Since it operates at elevated temperature normally above 700°C in SOFC mode, the sealing material should be stable at operating temperature and show a good flow tendency and adhesion property to the cell components during the sealing process, which normally is carried out above 850°C. Moreover, due to the high coefficient of thermal expansion (CTE) of the SOFC or RSOC components (> 110 × 10−7/°C), the sealing material should also have a high CTE to avoid possible thermal stress between cell components during operation.
Glass materials with a high glass transition temperature (Tg) and proper flow behavior at sealing temperature as well as a high CTE are thus required for SOFCs and RSOCs. Since the Tg of a glass system increases with structural strengthening components, which normally decrease CTE, it is difficult to find a proper glass system simultaneously having a high Tg and a high CTE. SiO2-BaO based glass systems such as SiO2-BaO-CaO-Al2O3, SiO2-BaO-Al2O3, and SiO2-BaO-B2O36-9) have been widely studied for SOFC sealing materials due to their high Tg and high CTE. Although their potential for sealing materials has been reported, there has been no systematic study on the effect of BaO content on the ternary system and its structural role to provide a high Tg with a high CTE at the same time has not been adequately discussed so far.
Thus, in this study, a SiO2-B2O3-BaO ternary glass system has been studied to find proper RSOC sealing materials and to understand the compositional effect of BaO on the thermal properties. With fixed content of SiO2=20, 25, and 30 mol%, Tg and CTE were monitored with BaO content and their respective structural change was inspected and discussed with Raman spectroscopy. The feasibility of Seal as a RSOC sealing material also has been examined.
2. Experimental Procedure
The glass compositions used in this study were 20SiO2-(80-x)B2O3-xBaO (SBB20x), 25SiO2-(75-x)B2O3-xBaO (SBB25x), and 30SiO2-(70-x)B2O3-xBaO (SBB30x) (in mol%), where × was varied from 45 to 60 mol%, respectively. High purity raw materials (> 99.9%) were weighed and thoroughly mixed with ball milling. Glasses were melted at 1400-1450°C for 1 h in an alumina crucible and then quenched in a brass mold, followed by annealing at 350-650°C for 5 h. The obtained glasses were ground into powder under 45 um in size.
Cylindrical samples (diameter of 12 mm) with 4 g of glass powder were formed by packing with a uniaxial press to examine the glass stability and flowability under heat treatment at 800°C and 900°C for 30 minutes. The Tg was determined by a differential scanning calorimeter (DSC-60, Shimadzu, Japan) and the CTE was determined in the range of room temperature to Tg by a thermo-mechanical analyzer (TMA-60H, Shimadzu, Japan). Raman spectroscopy (ARAMIS, Horiba Jobin Yvon, France) with an Arlaser source (514 nm center wavelength) was used to investigate the glass structure. Crystalline phase after heat treatment of the glass was determined by X-ray diffranction (Mini Flex 600, Rigaku, Japan) and the flowability of the glass was determined by a heating microscope (Misura(R) 3 HSM, TA Instruments, United States). A scanning electron microscope (SEM) equipped with an energy dispersive X-ray spectroscope (TESCAN, MIRALMN, Czech) was applied to examine the reaction between the glass and RSOC component.
3. Results and Discussion
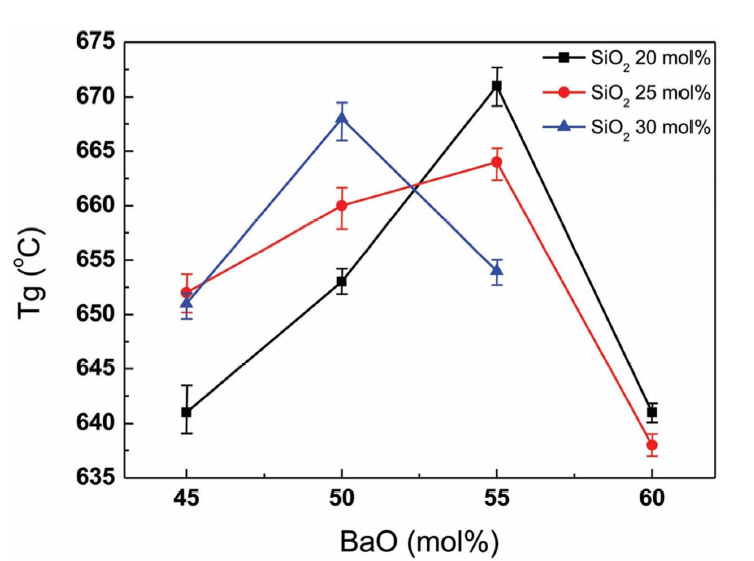
Under the same melting conditions, all compositions formed clear glasses except SBB3060 (x=60), which did not properly melt. The thermal properties of the obtained glasses were then examined. Fig. 1 exhibits the Tg change of SBB glasses with BaO content. As can be found in the figure, Tg increases with the addition of BaO up to 55 mol% for SBB20× and SBB25× and decreases thereafter at the fixed SiO2 content. Tg decreased from 50 mol% for SBB30× glasses. It is known that alkali- or alkaline earth-oxides (ROs) facilitate the formation of BO4 units and network connectivity within borosilicate glasses with increasing Tg and viscosity up to a given concentration, but generate non-bridging oxygens (NBOs) with decreasing Tg at higher concentrations. 10,11) Holbrook et al.8) inspected the topology and glass structure change according to BaO content of (BaO)x((B2O3)32(SiO2)68)100−x glasses and also observed an increase and decrease of Tg with an increase of BaO content. After Raman and volumetric analyses, as previously reported,8) they also suggested that the formation of BO4 tetrahedral units and charge compensation role of Ba-ions were responsible for the increase of Tg while the depolymerization role of BaO at a high concentration of BaO decreases the Tg. Thus, the change of Tg with BaO content can be elucidated in a similar way. The discrepancy of the Tg compared to the previous literatures12,13) is possibly due to the dissolution of Al2O3 from alumina crucible during the melting process. The effect of Al2O3 on Tg and viscosity on SiO2- B2O3-BaO system is currently under study.
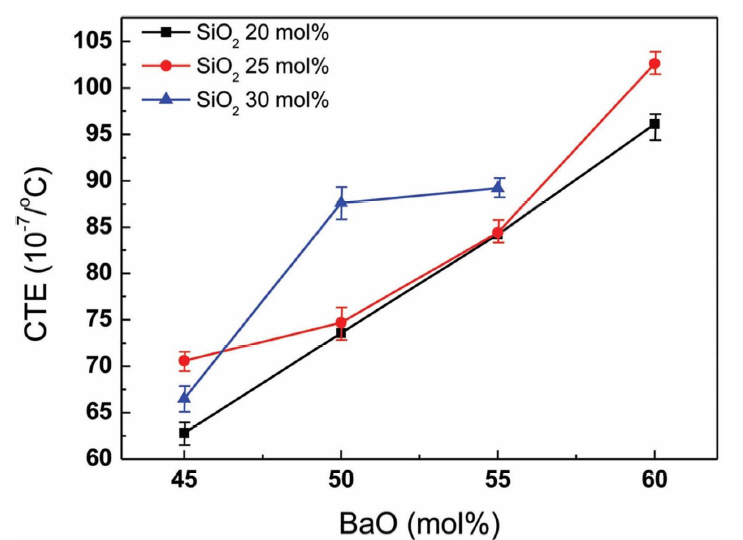
Figure 2 shows the CTE variation depending on the BaO content. As demonstrated in the figure, CTE increased with BaO content when SiO2 was fixed. Based on the structural role of BaO within borosilicate glass for Tg behavior, CTE is expected to decrease due to the increased BO4 units and network connectivity. However, CTE increased for all SBB glasses with BaO addition. It should be noted that the CTE is directly related to the bond strength of a solid and the replacement of B2O3 with BaO effectively reduces the average bond strength due to the weak bond strength of Ba-O (561.9 kJ/mol) compared to that of B-O (808.8 kJ/mol).12) Thus, it appears that the CTE of the present system has been more effectively determined by the average bond strength of components than the structural change of the glass. It should be also noted that, considering the high CTE of RSOC components, the CTE of the glass should be higher than 100 × 10−7/°C to reduce the possible thermal stress induced at the interface. According to the results obtained in Fig. 2, SBB2560 appears to be suitable for RSOC applications.
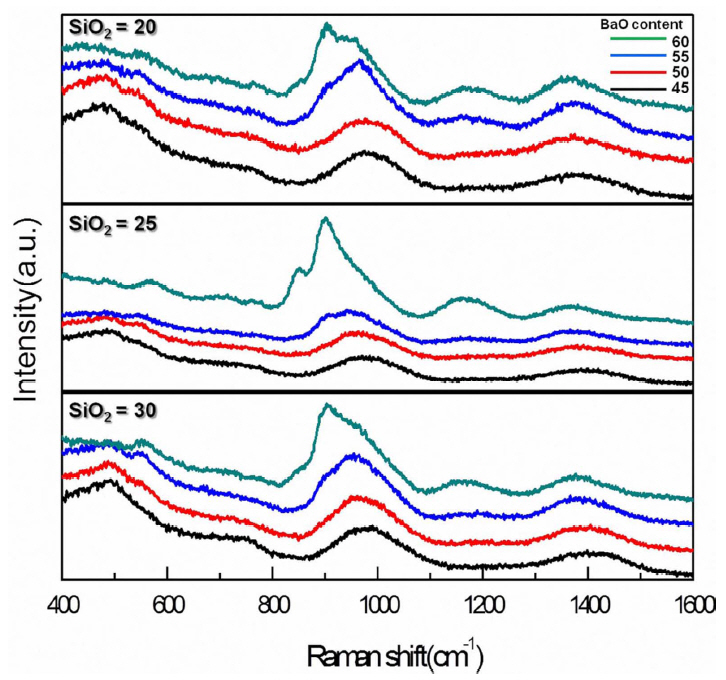
The structural change of the current glasses with BaO has been investigated with Raman spectroscopy. As shown in Fig. 3, all glasses show similar Raman peak evolution with the increase of BaO content for all the fixed SiO2 content. Raman shifted peak assignments are summarized in Table 1. As found in the figure, Raman peaks gradually varied up to 55 mol% of BaO. As BaO increased in lieu of B2O3, the peak at ~ 1040 cm−1 has been shifted to the lower energy side, implying the development of Q2 among the existing Q3 units, where Qn represents the [SiO4] tetrahedral unit with the number of bridging oxygens (n). The evolution of Raman peaks at 530 ~ 560 cm−1 at the expense of the peak at ~ 490 cm−1 indicates that the increase of [BO4] units replacing [SiO4] as BaO increases. These changes also have been observed with SiO2 content variation 8) and suggest that the formation of [BO4] structural units along with the structural variation of [SiO4] supporting the increase of Tg. However, it should be noted that the peak at ~ 900 cm−1 has been highly improved when BaO content was 60 mol%, indicating that the [SiO4] structural units exist mostly in Q1 and Q2 units. The peaks at ~ 840 and 1140 cm−1 represent the formation of orthoborates [BO33−] units and have been developed above 55 mol% of BaO. Along with the development of Q1 and Q2 units, this clearly supported the increased number of NBOs within the glass structure and the role of BaO as an efficient structure modifier above 55 mol%. Thus, it appears that BaO acts as a charge compensator, forming [BO4] units with modification of the SiO4 network below 55 mol%, and efficiently depolymerizes the glass network, forming NBOs above 55 mol% within the present glass system. These structural changes, as discussed earlier, elucidate the variation of Tg with BaO content.
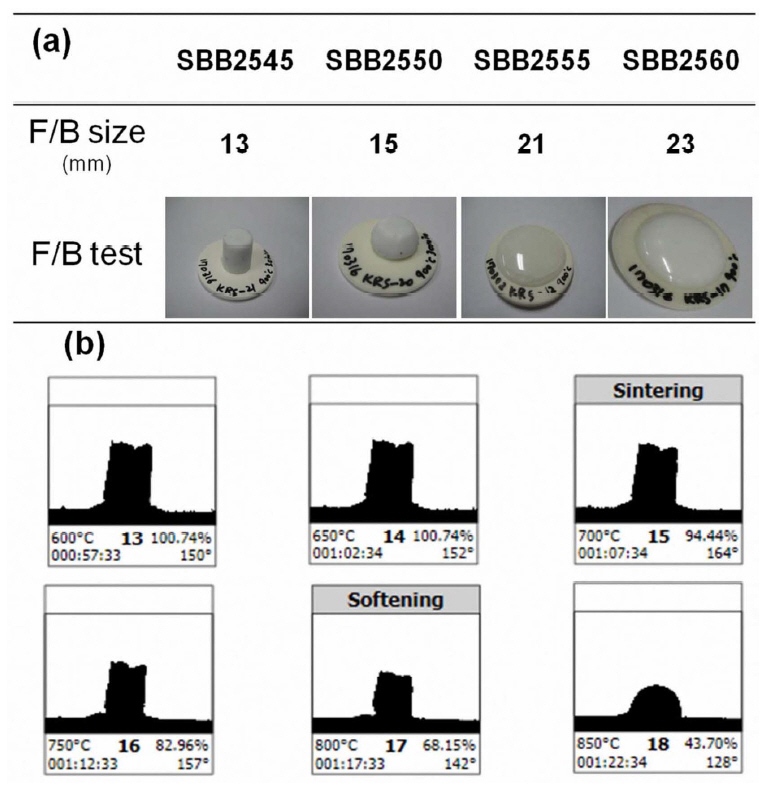
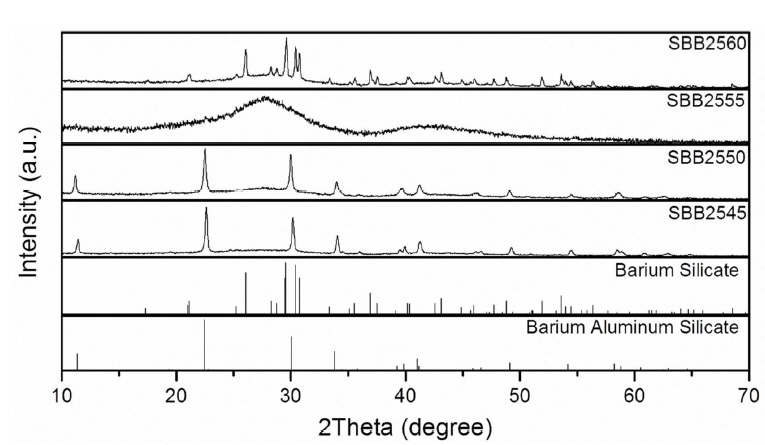
In order to be used as a hermetic sealing material for RSOC operating up to 750°C, it is desirable for the glass to show minimized viscous flow up to 800°C while it should guarantee a good flow at sealing temperature, for example, at 900°C. Fig. 4(a) shows the flow button test conducted at 900°C for SBB25× glasses with varying BaO content. As summarized in the figure, glasses with BaO content of 45 and 50 mol% showed no or limited flow while glasses with 55 and 60 mol% exhibited good viscous flow at the sealing temperature. Possible crystallization has been inspected with an X-ray diffractometer (XRD) and the results are depicted in Fig. 5. SBB2545 and SBB2550 glasses showed noticeable crystallization peaks corresponding to barium aluminum silicate (BaAl2Si2O8), which is expected to hinder the viscous flow at 900°C. Possible contamination of Al2O3 from the alumina crucible may facilitate the crystallization. Crystallization can help to control the viscous flow of the glass during the long term operation of the cell increasing mechanical strength, but the glass-crystal interface also can be a crack source. Further study is required for the effect of the crystallization. SBB2555 showed characteristic diffuse XRD patterns demonstrating its glass stability at the sealing temperature while SBB2560 presented crystallization peaks due to barium silicate (Ba2SiO4). Despite the crystallization, SBB2560 showed good viscous flow at the sealing temperature along with a high CTE for RSOC application. Thus, the viscous flow of SBB2560 glass with temperature variation has been examined using a high temperature microscope, as displayed in Fig. 4(b). Shrinkage of the glass powders started at 700°C but the visual viscous flow started from 800°C. The results clearly suggest that the glass can be a good candidate for SOFC or RSOC operating at 700 ~ 750°C along with a good viscous flow property at the sealing temperature and high CTE.
The binding property of the SBB2560 glass has been inspected with YSZ coated with NiO-YSZ substrates with sintering temperature at 900°C for 30 min. The glass was thoroughly mixed with ethyl cellulose-based organic vehicle to make a paste with a mixing ratio of 7:3 in wt.%. The organic vehicle was removed at 450°C for 1 h during the heat treatment process for sealing. As can be found in Fig. 6, the intermediate layer between the glass and substrate is the YSZ coating layer of the NiO-YSZ substrate. And the glass bonded well to the NiO-YSZ layer with minimized pores trapped. No crystallization or a noticeable reaction layer was observed. Elemental analysis via SEM-EDS revealed that the elements for sealing glass and NiO-YSZ layer were well confined within each material, clearly supporting the restricted reaction between them as well as the practical feasibility of the present glass as a RSOC sealing material.
4. Conclusions
SiO2-B2O3-BaO ternary glasses were synthesized with varying BaO and B2O3 content at fixed SiO2 concentrations. When BaO content was increased at the expense of B2O3, CTE increased while Tg showed a maximum at around 55 mol% of BaO. The formation of [BO4] tetrahedral units strengthening the glass network was considered to be responsible for the increase of Tg and the effective network breakage generating NBOs, Q1 and Q2 units of [SiO4] and [BO33−] units were responsible for the decrease of Tg at high BaO content. The increase of CTE in spite of network strengthening with BaO is attributed to the decrease of bond strength due to B-O replacement with Ba-O. SBB2560 showed a high CTE with reasonable flow characteristics at the operating and sealing temperatures. It also showed a good adhesion property without crystallization and reactions with YSZ coated with NiO-YSZ, demonstrating its feasibility for RSOC applications. However, further study is required to improve the glass stability and further examinations such as studying the binding properties with other RSOC components and long-term leak tests also should be carried out for practical application of the glass materials.