1. Introduction
Over the past few decades, lanthanide orthovanadate (LnVO4) has attracted a considerable amount of attention from specialists and scholars given its various technological applications in areas such as catalysts,1) polarizers, laser host materials,2) phosphors3) and luminescent materials. 4,5) Generally, the larger Ln3+ ion prefers to form a monazite structure owning to its higher oxygen coordination number of 9 compared to 8 for the zircon type. In contrast to other forms of LnVO4, LaVO4 crystals can crystallize in two types of polymorphs,6) i.e., a tetragonal (t-) zircon7) type and a monoclinic (m-) monazite8,9) type, with the latter always noted as a thermodynamically stable state. Therefore, Eu3+-doped LaVO4 can be formed as either a monazite or a zircon type of structure.
Recently, much effort have been expended to fabricate LaVO4-based phosphors with excellent luminescent properties, 10-12) and all these efforts have brought promising luminescence applications. Several reports on LaVO4:Eu3+ nanocrystals have been published. However, luminescence studies of both m- and t-LaVO4:Eu3+ nanocrystals remain insufficient.
2. Experimental Procedure
2.1. Sample preparation
All of the chemical reagents used here were of analytical grade, and all were used as received without further purification. Ethylene glycol (EG) was used as a capping agent and a reaction medium. The various lanthanide salts used were europium nitrate (Eu(NO3)3·6H2O, 99.99% Sinopharm), lanthanum nitrate (La(NO3)3·6H2O, 99.99% Sinopharm) and sodium vanadate (Na3VO4), 99.99% Aladdin).
2.2. Synthesis of LaVO4:Eu
In a typical procedure, an amount of x mL of EG was initially dissolved in (16-x) mL of deionized water. Then, La(NO3)3 (y mmol) and Eu(NO3)3·6H2O ((1-y) mmol) were added into the obtained solution to form a chelate lanthanum complex. Subsequently, 1 mmol of Na3VO4 in 6 mL of deionized water was added into the solution in a dropwise manner under vigorous magnetic stirring. The pH value of the solution was adjusted to 3, 5, 7, and 9 with a solution of hydrogen nitrate and sodium hydroxide. The resultant solution was then transferred into a 35 mL autoclave and maintained at 180°C for 12 h. Finally, the resulting mixture was cooled at room temperature, separated by centrifugation, and washed with deionized water and ethanol several times. The final product was dried at 80°C in air for 6 h, and the dried sample was used for characterization.
2.3. Characterization
Powder X-ray diffraction (XRD) patterns were examined on a MAC Science Co. Ltd. MXP 8 AHF X-ray diffractometer with monochromatized Cu-Kα radiation (λ = 1.541874 Å). Field-emission scanning electron microscope (FE-SEM) measurements were carried out at a voltage of 10 kV. The photoluminescence (PL) spectra were recorded on a Hitachi F-4600 fluorescence spectrophotometer with a 150 W xenon lamp as the excitation source at room temperature.
3. Results and Discussion
3.1. Phase and morphology
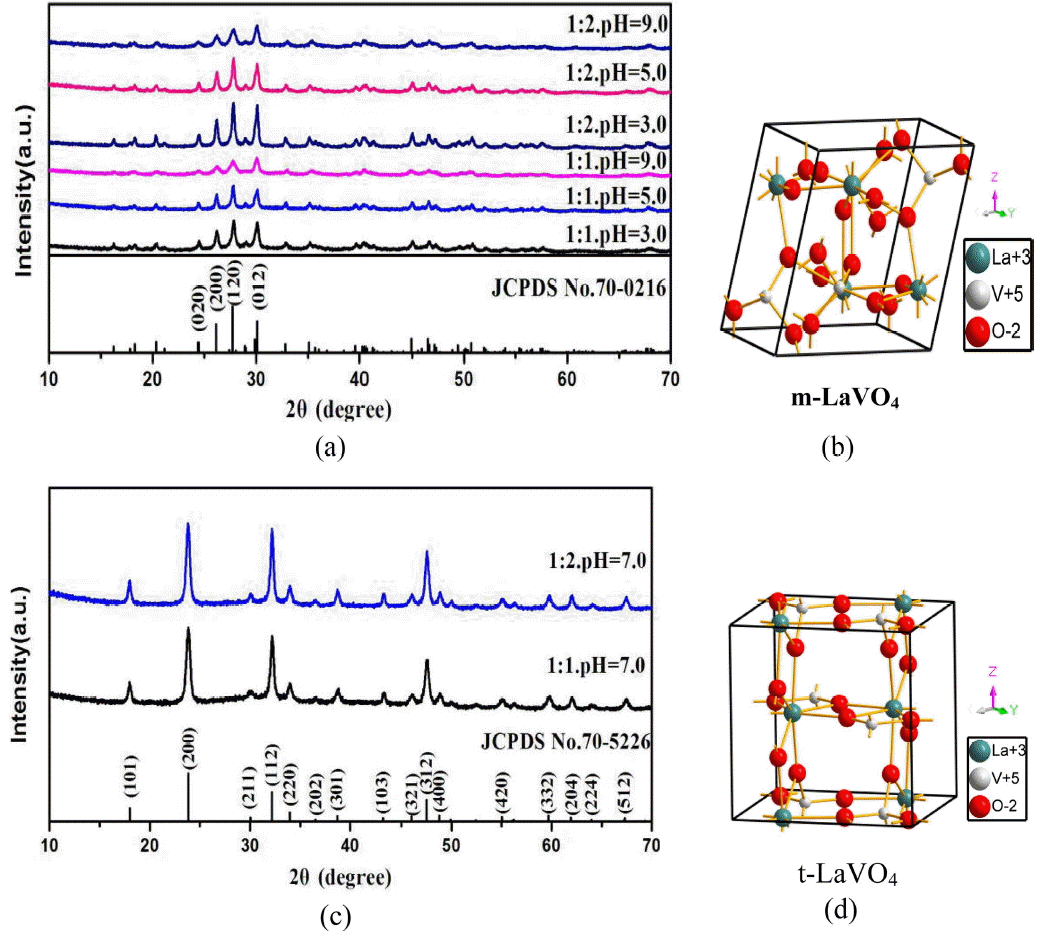
Figure 1 shows the X-ray diffraction (XRD) patterns of LaVO4:Eu3+ nanocrystals synthesized at 180°C for 12 h at pH values of 3, 5, 7, and 9, as well as the crystal structures of m-LaVO4 and t-LaVO4. From Fig. 1(a), it can be seen that all of the diffraction peaks in the six patterns can be indexed to m-LaVO4 (JCPDS No. 70-0216) without a trace of any other phase when the pH value was 3, 5 and 9, indicating that the six samples were of high purity. Moreover, it was clear that when the ratio of deionized water was 1 : 2 and the pH value was 3.0, the diffraction peaks of the sample were sharper than in the other cases, indicating enhanced crystallinity of the products. This is important for phosphors, as high crystallinity improves the luminescence. Compared to Fig. 1(c), all of the diffraction peaks in the other two patterns could be indexed to t-LaVO4 (JCPDS card No. 70-5226) without traces of other phases when the pH value was 7, which also indicate high purity of the two samples.
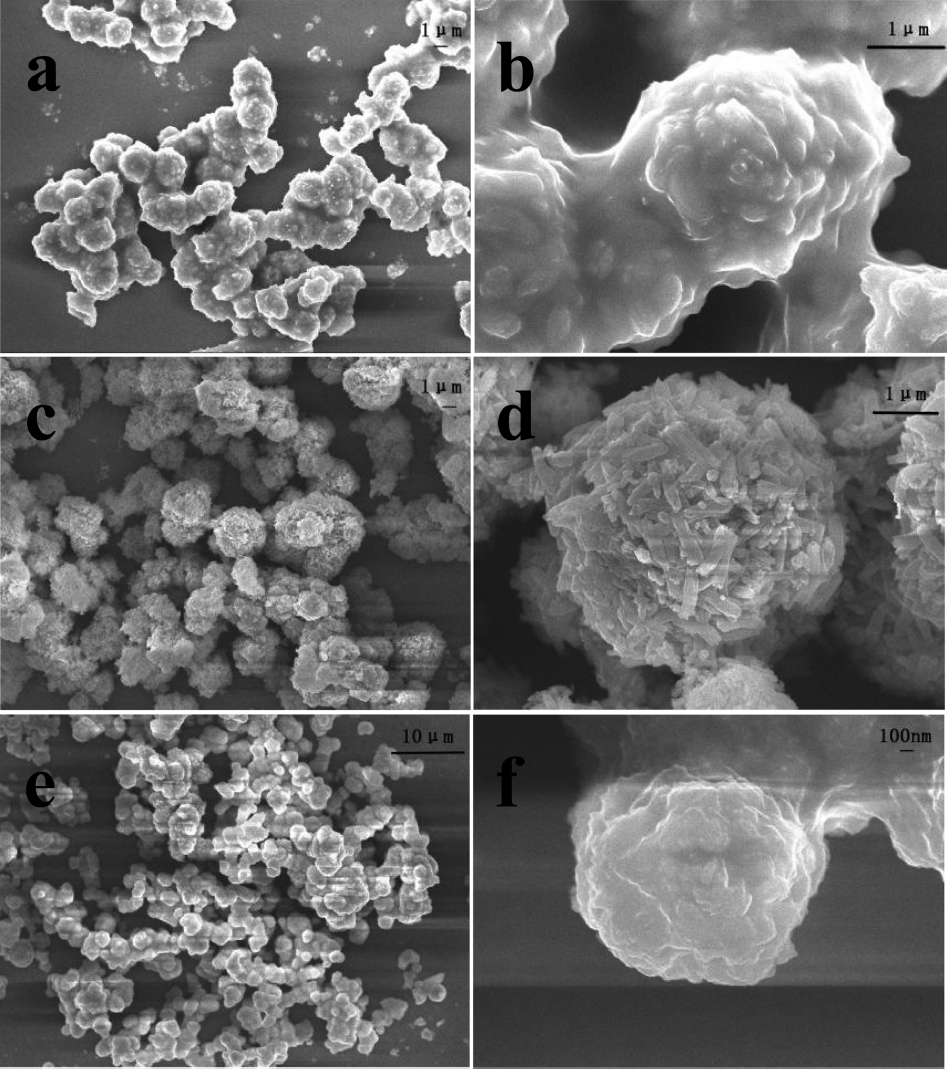
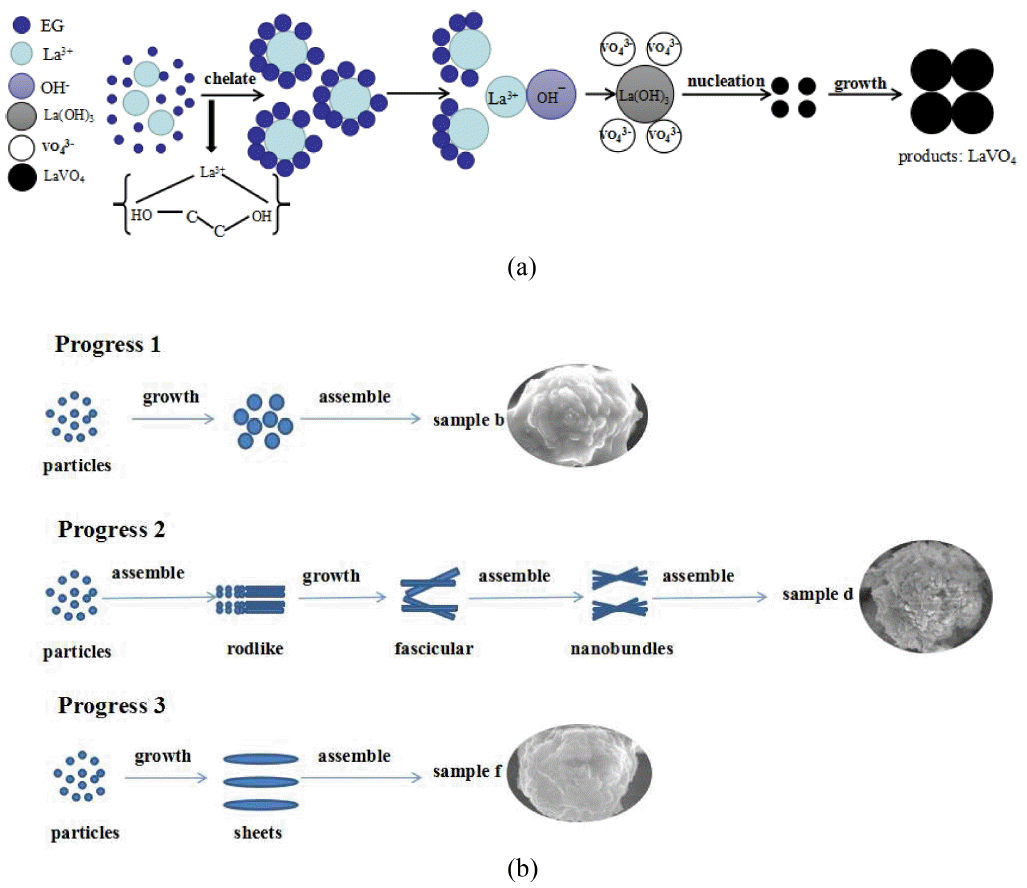
Figure 2 shows SEM images of LaVO4 prepared at different ratios of EG when the pH and the mole percent of Eu3+ were known. Fig. (a) and (b) show that for the m-LaVO4 product, the average diameter of the particle was about 2 μm, with many spherical particles. In addition, it was noted that every spherical particle was assembled with numerous smaller particles. Comparing Fig. (c) and (d) shows diverse spherical particles with better dispersibility and an average diameter of approximately 4 μm. It was clear that the spherical particles consisted of fascicular nanowires. Similarly, Fig. (e) and (f) show spherical particles with an average diameter of 1 μm assembled into nanosheets. In conclusion, it was not difficult to find that the dosage of EG played a crucial role in the features of the products by affecting their growth process. When the ratio of EG/H2O was 0.5, the product consisted of many spherical particles consisting of smaller particles, and with an increase in the EG/H2O ratios to 1 and 2, the products were spherical particles which consisted of wires and sheets, respectively. Here, La3+ ions form a chelate liquid with EG initially, after which La3+ ions are released one by one to combine with OH− to generate nanosized La(OH)3, which served as nucleation sites. Finally, VO43− species could be attached to the surface of La(OH)3 grains, resulting in the nucleation and growth of LaVO4,15-17) as shown in Fig. 3(a). Meanwhile, different amounts of EG led to different coordination numbers of the chelate liquid, resulting in different crystal growth mechanisms to form different shapes. Fig. 3(b) shows the possible growth process of LaVO4 using different dosages of EG as an additive.
3.2. Luminescent properties of LaVO4:Eu
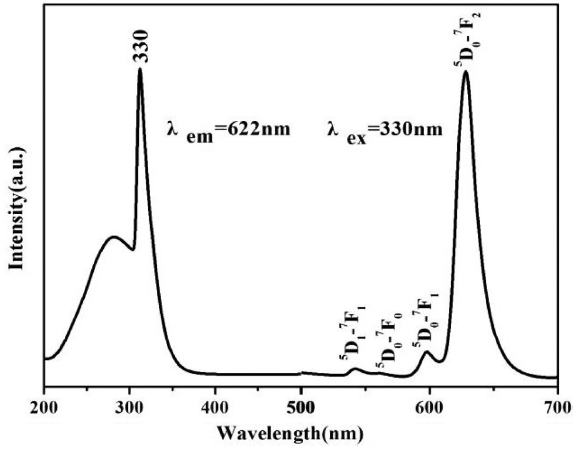
Figure 4 shows the excitation and emission spectra of the t-LaVO4:Eu (5 mol%) nanocrystals. All of the excitation and emission spectra were similar in terms of their positions but had intensity levels different from that of m-LaVO4. It was also noted that the excitation spectrum of the as-prepared t-LaVO4:Eu nanocrystals consisted of an absorption band with a maximum at 330 nm; the strong absorption was due to a charge transfer from the oxygen ligand to the vanadium atom within the VO43− group ions.18) In terms of molecular orbital theory, this is in accord with transitions from the 1A2(1T1) ground state to the 1A1(1E) and 1E(1T2) excited states of VO43−.
The emission spectrum exhibited several groups of emission peaks from 500 to 700 nm which were ascribed to the 5D1-7F1 and 5D0-7FJ (J = 0, 1, 2) transitions of Eu3+ ions.19,20) In addition, crystal field splitting of the Eu3+ 5D0-7F2 transitions was predominant, indicating that the LaVO4:Eu3+ had good crystallinity. Thus, the excitation and emission process included three major steps.21) The first of these was the absorption of energy by VO43− groups, after which the energy was transferred to Eu3+ from VO43−, with the excited Eu3+ ions finally producing red emission.22,23)
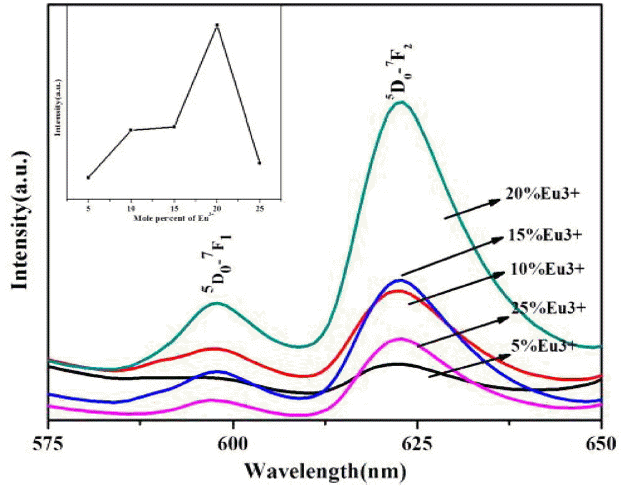
Figure 5 shows the emission spectra of m-LaVO4 prepared at an identical pH but doped with different dosages of Eu3+. It can be seen in Fig. 5 that the emission intensity was enhanced with an increase in the mole percent of Eu3+. When the mole percent was 20%, the product had the highest emission intensity. However, at greater levels the intensity was reduced due to concentration quenching caused by the higher concentration. Similarly, this law applies to other products prepared under different conditions.
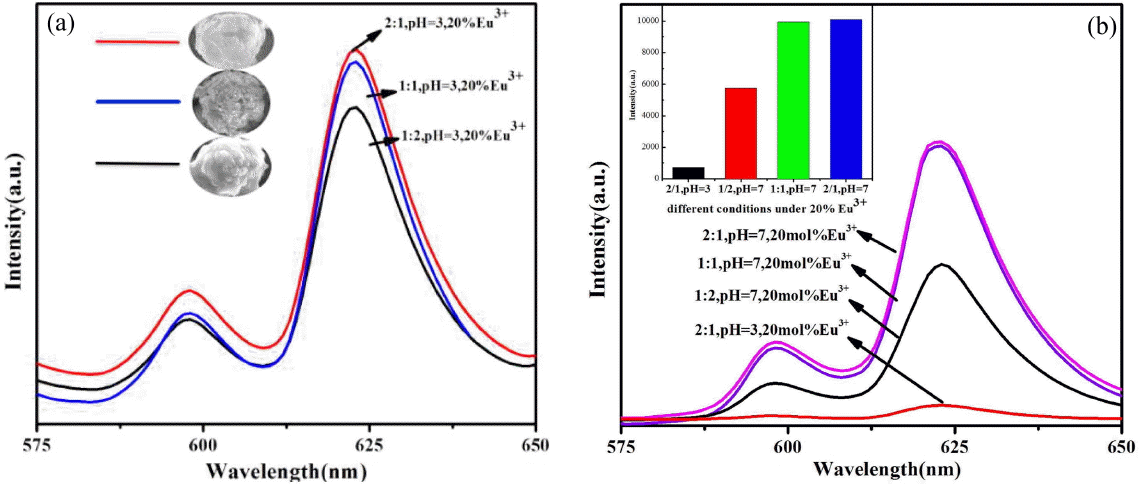
Figure 6(a) shows the emission intensity of m-LaVO4 prepared at the same pH but with different dosages of EG when the doped Eu3+ amounted to 20%. It is clear that the relative intensity of m-LaVO4 was enhanced gradually with an increase in the EG, and the intensity was highest when ratio of EG was 2 : 1. It is known that the degree of symmetry in the vicinity of Eu3+ ions and Eu-O covalency is affected by the site asymmetry, electronegativity, and covalency of the ligand atoms.24) Furthermore, the symmetry or integrity of the crystal structure contributes to the fluorescence, for which higher symmetry or integrity of the crystal structure leads to higher intensity of the fluorescence. We can clearly observe in Fig. 6(a) that when the ratio of EG was 2 : 1, the products were spherical particles with assembled by sheets had the highest intensity, indicating a better crystal structure. Logically, by reduced the ratio of EG to 1 : 1 and 1 : 2, a lower crystal structure results, causing the products to consist of smaller nanowires and particles with relatively low intensity of the fluorescence.
Figure 6(b) shows the relative intensity levels of t-LaVO4 and m-LaVO4. As is shown the luminescence properties of t-LaVO4 are better than those of m-LaVO4, confirming that t-LaVO4 is a better host for Eu3+. In fact, for t-LaVO4:Eu3+, there were several key bridges of La/Eu-O-V with a maximum angle of 153° (Fig. 7(a)) in every lanthanum or europium center, which were better for the overlap of the wave functions of vanadium and lanthanum such that the energy transfer was greatly improved. In comparison, in the m-LaVO4:Eu3+, there was only one key bridge with an angle of 153°; the other key bridge angles were smaller (Fig. 7(b)), in contrast to the energy transfer. Thus, the luminescence properties of t-LaVO4 were superior to those of m-LaVO4.
4. Conclusions
In summary, pure m-LaVO4:Eu3+ and t-LaVO4:Eu3+ nanocrystals were prepared by an EG-assisted hydrothermal method with regular shapes. A series of controlled experiments showed that the pH value of the mixed solution, the volume ratio of EG/H2O and the dosage of the doped Eu3+ all had an important effect on the size and shape of the final products. The PL results showed that Eu3+ ions doped into t-LaVO4 led to better luminescence properties than m-LaVO4 due to its special structure. Thus, t-LaVO4 is shown to be a new and inexpensive candidate for luminescence materials with potential applications in lighting, displays, and biological detection. All of these results not only expand our knowledge of the luminescence properties of lanthanide orthovanadates but also contribute to a better understanding of the principles of the crystal growth of these materials.