1. Introduction
Ultra-high temperature ceramics (UHTCs) are used as structural materials or coating materials in extreme environmental conditions such as ultra-high temperatures and corrosion because they have high melting points, high mechanical strength in an ultra-high temperature environment, abrasion resistance, and environmental resistance.1) Tantalum carbide (TaC) is a kind of UHTCs and it is one of Group V transition metal carbides. It has melting points of 3300°C or higher degrees, and in the case of TaC~0.89, which is TaC with a deficit of carbon, the melting point may reach about 4000°C in.2) It exhibits high stability to the melting point without any phase changes, high hardness, high strength, and excellent thermal shock resistance, so it can be applied to the condition where thermomechanical loading is applied, wear-resistant structural parts, and components for thermal heat protection in the form of a composite material or coating material. Recently, due to its properties such as resistance to chemical attack, excellent compatibility with a heat-resistant metal, and very low gas permeability as well as high melting points, it is being considered for a coating material for the support portion of sapphire growth crucibles or for SiC epi susceptors.3)
TaC can be formed by methods such as hot isotatic pressing( HIP),4,5) hot pressing(HP),6) the molten salt method,7) spark plasma sintering (SPS),8) plasma spraying,9) laser ablation deposition,10) sputtering,11) and chemical vapor deposition (CVD).12-16) When TaC is deposited using the CVD process, techniques such as metal-organic CVD (MOCVD),12) hot-filament CVD,13,14) and hot-wall CVD15-17) are used depending on source materials and heating methods.
In order to use it as the coating material for a semiconductor susceptor or for a crucible for the single crystal growth for light emitting diode (LED) application, the impurity concentration of TaC coating layers is required to be very low. Thus, CVD method is suitable for this purpose, and hot-wall CVD is favorable for uniform deposition of TaC onto large components. Therefore, in this study, TaC was deposited by the hot-wall CVD method using TaCl5 as the source material, and parameter studies and optimization were carried out in order to obtain excellent TaC coating layers.
2. Experimental Procedures
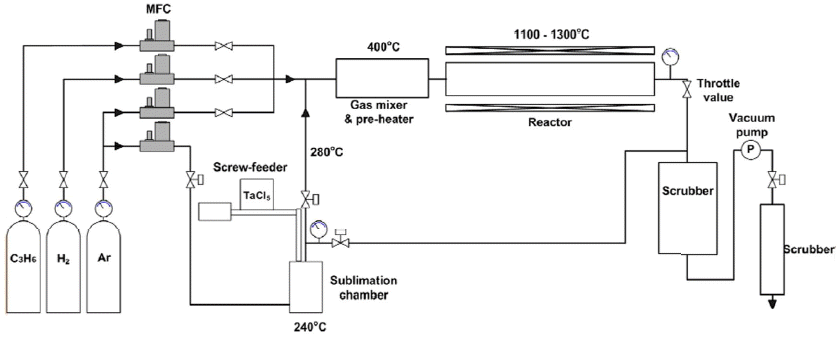
TaC was deposited using TaCl5-C3H6-Ar-H2. The low pressure chemical vapor deposition device used for TaC deposition is shown in Fig. 1. Deposition experiments were carried out placing a high purity graphite disc plate with the diameter of 50 mm and thickness of 1 mm on an inclined graphite plate with an inclination of 7°. The source material was high purity TaCl5 powder (> 99.99%, STREM CHEMICALS, INC). In order to maintain a uniform partial pressure of TaCl5 during the deposition process, a constant amount of the powder was continuously fed into the sublimator using a screw-driven powder feeder. In order to evaluate the characteristics of TaCl5 powder feeding, the supply amount of the powder according to the rotational speed of the feeder screw and the amount of the powder fed into the sublimation chamber according to time were measured using a scale in real time, and the optimal feeding rate of powder was determined on the basis of the results. After 90g of TaCl5 was filled into a cylinder for TaC deposition, the vacuum state was maintained long enough to remove the internal air, and then the powder was fed into the sublimation chamber evenly for 30 minutes at the rotation speed of the screw feeder of 3.9 rpm.
In addition, thermogravimetric analysis (TGA) was carried out to conduct evaluation of the sublimation behavior of the powder, and mass changes were measured by getting high purity helium, a kind of inert gas, to flow in at the flow rate of 50 sccm. After it was transformed into a gaseous state at 180°C, which is the sublimation temperature of crystallized TaCl5, through TGA analysis, TaCl5 gas was made to flow into the reactor using Ar as a carrier gas at the flow rate of 800 sccm.
C3H6 was used as the source gas of carbon. By changing the amount of C3H6 while maintaining a constant supply amount of TaCl5, the ratio of Ta/C was adjusted. TaC deposition was performed at 1100°C and 1300°C. Deposition pressure was kept constant at 6.7 kPa using a throttle valve, and diluent gas H2 was made to flow in so that the total flow rate could be 3000 sccm. The conditions of TaC deposition are summarized in Table 1.
The equilibrium compositions in H2-C3H6-TaCl5 system were calculated using the HSC chemistry ver. 6.0. The changes in equilibrium compositions with the amount of diluent gas H2 were estimated under the conditions of TaCl5:C3H6 = 3 : 1, 1300°C, and 0.1 bar.
3. Results and Discussions
3.1. Sublimation and Feed Behavior of TaCl5 Powder
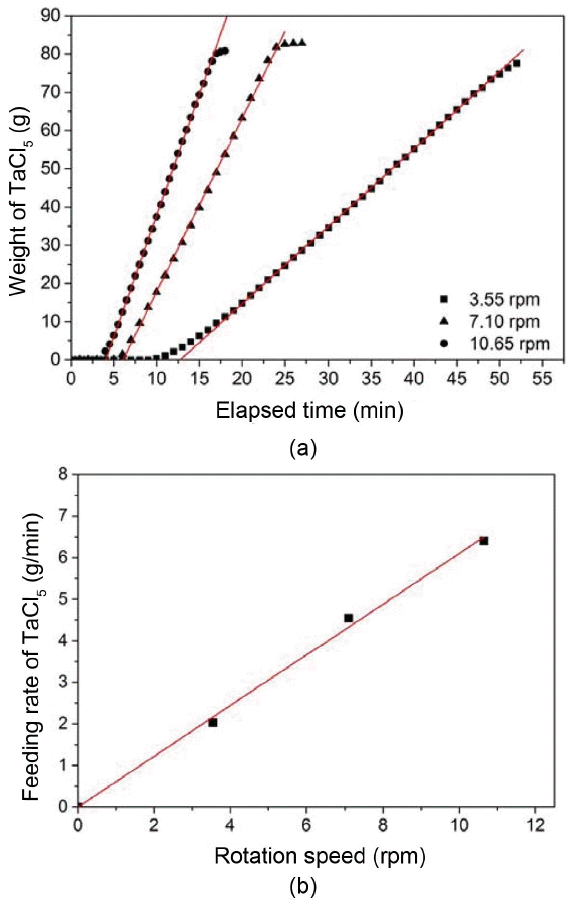
When carbides are deposited using solid powder materials, the amount of sublimated powder should be supplied at a constant partial pressure to obtain coating layers having a uniform phase. Therefore, when sublimation occurs after feeding all powder before deposition, the adjustment of sublimation temperatures and pressure of the sublimation chamber is an important factor. However, as deposition proceeds, the stoichiometry of coating layers depending on deposition time can be changed if the sublimation of the powder does not proceed constantly due to problems such as consumption or agglomeration of the powder.18) In order to solve this problem, a powder feeding system which is a type of screw feeder was used in order to induce sublimation supplying a constant amount of powder during deposition. Fig. 2 shows the supply amount of the powder as a function of the rotational speed of the screw of the powder feeder and elapsed time. At the beginning of powder feeding, powder is not fed until it reaches the sublimation chamber. It increases in proportion to the rotation speed of the screw. Subsequently, it can be seen that a constant amount of the powder is fed constantly without fluctuation depending on time, and linearity is maintained even though the rotational speed of the screw is changed. The feeding rate of the powder is found to change linearly as the rotation speed of the screw increases, and thus, the amount of the fed powder during any deposition time can be determined and it is precisely adjustable.
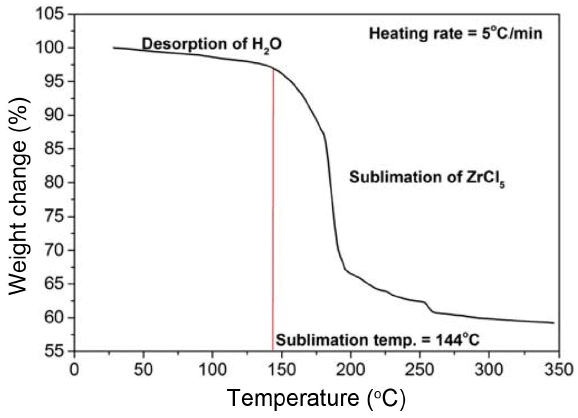
The weight changes depending on the temperatures of TaCl5 powder were measured using TGA and the results are presented in Fig. 3. TaCl5 powder reacts very rapidly with moisture at room temperature. Although the TGA experiment was conducted in a He environment, the powder was exposed to the air while the sample was prepared and while the powder was fed into the TGA holder, which may have resulted in the adsorption of H2O. For this reason, the decrease in mass was observed because of the desorption of water during the initial temperature increase. The decrease in mass due to the sublimation of TaCl5 powder used in the experiment is believed to start at about 150°C. A very high sublimation speed is observed in the vicinity of 180°C.
3.2. Deposition Behavior of Ta-C
The chemical vapor reactions at a high temperature of TaCl5 and C3H6 proceeds in the following steps.19)
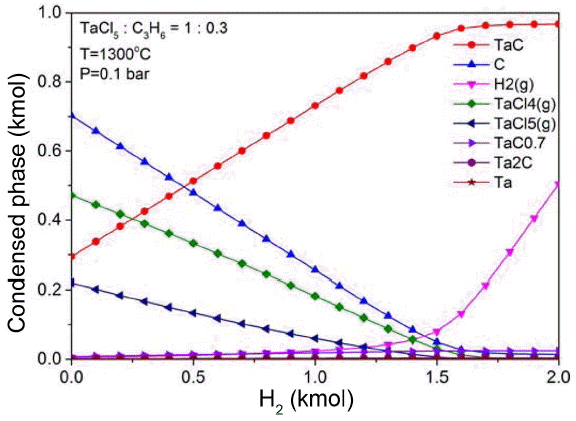
TaC compounds are produced by the reaction of Ta and C formed as a result of the decomposition of C3H6 and TaCl5 at a high temperature. If there is an excessive amount of Ta, then Ta6C5, Ta4C3, Ta2C, etc. may be formed, and if there is a more amount of Ta, the formation of metallic Ta is promoted. At this time, H2 is generated as a result of decomposition of C3H6, and participates in the decomposition reaction of TaCl5. Although C3H6 has a high production rate of reactive carbon concentration by pyrolysis at a temperature between 1100 °C and 1300°C,20) the amount of H2 generated by decomposition of C3H6 is not sufficient to decompose TaCl5 considering the experimental conditions, as shown in the Fig. 4. Therefore, in this study, the degradation of TaCl5 was allowed to occur sufficiently regardless of the changes in the amount of C3H6 by using H2 as diluent gas. Under the conditions of TaCl5:C3H6 = 3 : 1, 1300°C, and 0.1 bar, the degradation of TaClx gas was found to increase as H2 increased, and TaClx gas was decomposed almost completely in H2 of about 1.5 kmol, demonstrating a high efficiency of TaC.
Figure 5 shows the change in the microstructure of TaC coating layers depending on the powder feeding method. When sublimation is induced by feeing all the powder into the sublimation chamber at the same time before the deposition experiment, TaCl5 vapor pressure in the sublimation chamber is maintained in the saturated state at an early stage of deposition. However, the vapor pressure may be continuously reduced at a later stage of deposition as the amount of TaCl5 decreases gradually.18) Yet, if the powder is continuously supplied using a screw-driven feeder during the deposition process, a constant vapor pressure is always maintained. Therefore, with respect to the thickness of TaC coating layers, when it is deposited using a screw-driven feeder, the thickness of coating layers is found to increase approximately by two times due to the efficient supply of TaCl5.
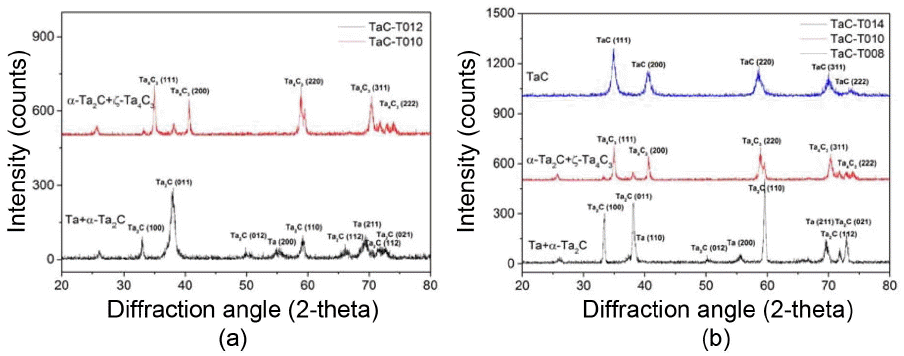
Figure 6 shows the results of the XRD analysis of two different TaC coating layers formed by different powder feeding methods and the Ta-C phase diagram. When a screw-driven feeder was not used, TaC coating layers were composed of Ta, α-Ta2C, and TaC, as shown by the results of the XRD analysis in Fig. 6(a). However, if the C/Ta ratio is lower than 1 at 1300°C, Ta+α-Ta2C, α-Ta2C+TaC1-x, or TaC1-x, which are Ta-rich phases, is formed as the C/Ta ratio increases, as seen in the Ta-C phase diagram of Fig. 6(b).2) If the C/Ta ratio is greater than one, a TaC+graphitic carbon phase is produced. Thus, the fact that these three different phases were detected at the same time indicates that the ratio of C3H6/TaCl5 continuously changed during the deposition process. In other words, as the deposition proceeds, the amount of TaCl5 powder decreases, thus, the partial pressure of the TaCl5 gas in the sublimation chamber is gradually reduced, and as a result, the ratio of C3H6/TaCl5 is gradually decreased. Therefore, it can be seen that the initial Ta-rich phase, the Ta+α-Ta2C mixture is turned into α-Ta2C+TaC1-x or TaC at a late stage of deposition. However, if the powder is continuously supplied using a screw-driven feeder under the same deposition conditions, then the partial pressure of TaCl5 is kept constant during the deposition process, so Ta+α-Ta2C phase, a stable phase observed when the ratio of C/Ta is between 0 and 0.4, is maintained, and as a result, an abnormal combination of phases is not observed. These results show that the use of a screw feeder led to the improvement in the uniformity and efficiency of the feeding rate of TaCl5, and thus the phases of coating layers became uniform and the thickness of coating layers increased.
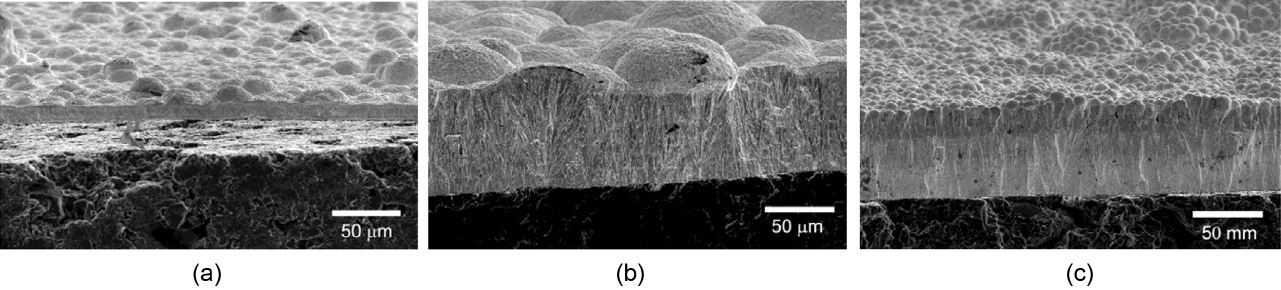
Figure 7 shows the changes in the microstructure of coating layers according to the deposition temperature and change of C3H6/TaCl5 ratio. As shown in Fig. 7(a) and (b), if it is deposited for 30 minutes, the thickness of the coating layer increases from about 11 μm to 65 μm as the deposition temperature increases from 1100°C to 1300°C. In addition, the α-Ta2C+ζ-Ta4C3 phase formed at 1300°C was transformed into Ta+α-Ta2C at 1100°C as shown in Fig. 8(a). However, phase changes depending on the change of temperature do not occur at a temperature below about 1450°C, as seen in the phase diagram of Fig. 6(b). Therefore, these phase changes may be attributed to the fact that as the temperature was lowered, the high-temperature thermal decomposition rate of C3H6 decreased to a greater extent than that of TaCl5. As a result, the surplus Ta generated by the decomposition of TaCl5 was unable to react with C, and was deposited with the α-Ta2C.
Deposition was performed varying the ratio of C3H6 and TaCl5 in order to find the optimum deposition conditions at 1300°C. When the amount of C3H6 was changed maintaining a constant amount of TaCl5, variation in the thickness of coating layers was hardly observed. On the other hand, when the ratio of C3H6/TaCl5 was low (Fig. 5(b)), Ta+α-Ta2C was formed and it was observed to have a smooth surface. In addition, even when a bending stress was applied to coating layers, they were not broken but plastic deformation occurred readily. This means that the ratio of Ta was considerably high. However, the phase was found to change into α-Ta2C+ζ-Ta4C3 and TaC1-x as the ratio of C3H6/TaCl5 was increased. When it changed into the combination of Ta2C and TaC, the surface had a dome top structure and the fracture surface had a columnar structure.
Figure 9 shows the results of the XPS and Raman analysis of TaC coating layers having an TaC1-x single phase. TaC was found to have four major XPS peaks. They were the peaks corresponding to TaC and TaxOy, and these results may indicate that a very small amount of air entered the CVD system and the peaks of oxides were observed together.21) Among the analyzed peaks, the peaks of Ta 4f5/2 and Ta 4f7/2 corresponding to TaC were found to have the binding energy of approximately 25.3 eV and 23.5 eV, respectively and they nearly coincided with TaC peaks with the stoichiometric ratio.21) In addition, when Raman analysis was conducted on a cross-section of TaC coating layers in the thickness direction, the results showed that the peaks of acoustic branches, A1 (110 cm−1) and A2 (166 cm−1) and the peaks of optical branches, O1 (606 cm−1) and O2 (652 cm−1), which are caused by the carbon deficit, were hardly detected. Thus, the amount of carbon deficit is considered to be very low.22) Moreover, the disorder-induced peak (1350 cm−1) and graphite peak (1580 cm−1), which are caused by C when TaC + C phase is formed, are not observed either. Therefore, it was concluded that when TaC coating layers were formed using the low-temperature chemical vapor deposition (LPCVD) method, single phase with excellent stoichiometry was formed.
4. Conclusions
By using the low pressure chemical vapor deposition (LPCVD) method in TaCl5-H2-Ar-C3H6 system, TaC was deposited on graphite. If deposition is achieved by sublimation using a chloride-based powder, changes in the sublimation amount depending on the deposition time are likely to occur. Therefore, in order to obtain TaC with a uniform phase by maintaining a constant partial pressure of TaCl5 during deposition, TaCl5 powder was fed into the sublimation chamber using a screw-driven powder feeder. The changes in the feeding time of TaCl5 powder and in the rotational speed of the feeder were evaluated to have extremely high linearity. The application of a screw feeder resulted in an approximately twofold increase in the deposition rate and improvement of phase homogeneity. As the deposition temperature was increased, the deposition rate of TaC dramatically increased, and it was transformed into Ta-C phase, which contains more Ta. By optimizing the ratio of C3H6/TaCl5 at 1300°C, it was possible to obtain near-stoichiometric TaC, and the measurement results showed that phase homogeneity was excellent in the direction of the thickness of TaC coating layers.